Simon Cotton looks at compounds in the news or relating to our everyday lives.
In this issue: xenon dioxide
Missing compounds
Two Canadian chemists, David Brock and Gary Schrobilgen, have just filled in one of the gaps on the short-list of 'missing compounds' by preparing xenon dioxide1. XeO3 and XeO4 had been known for years, but until now, no one had succeeded in making XeO2.
Even though xenon is the most reactive noble gas, fluorine is the only element that chemists have made to react with it, so XeO2 was made by an indirect route: the hydrolysis of XeF4 using ice-cold water.
XeF4 + 2H2O → XeO2 + 4HF
Shape and structure
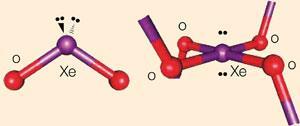
At room temperature, XeO2 is a yellow-orange solid, but unlike the other two oxides, which are made of individual molecules, XeO2 has a polymeric structure in which xenon is bonded to 4 oxygen atoms in a square-planar arrangement. Each oxygen atom is shared between 2 xenons, so the compound could be described as XeO4/2.
Forming four single bonds to the oxygen atoms gives xenon four electrons to add to the eight of a Group 0 element, so there are six electron pairs arranged octahedrally, with the lone pairs mutually trans-, minimising repulsions.
Missing xenon
Scientists have long known that the amount of xenon in the earth's atmosphere is well below the amount expected. Working with diamond-anvil cells (to generate high pressures) at high temperature, scientists at the University of California, Berkeley, established in 1997 that the xenon was not dissolving in the molten iron at the core of the earth.2
However, in 2005 a team led by Chrystèle Sanloup, then at the Université Pierre et Marie Curie, Paris, used x-ray diffraction to show that at high temperatures some xenon substituted for silicon in quartz (SiO2).3 Theoretical calculations support this idea, so that this implies that 'missing' xenon could be tied up deep in the earth's crust.
Proving the existence of XeO2 gives credence to this theory, besides being an important scientific discovery in its own right.
References
- D S Brock and G J Schrobilgen, J. Am. Chem. Soc., 2011, DOI: 10.1021/ja110618g
- W A Caldwell et al, Science, 1997, 277, 930 (DOI: 10.1126/science.277.5328.930)
- C Sanloup et al, Science, 2005, 310, 1174 (DOI: 10.1126/science.1119070)









No comments yet