Joe Ogborn takes a tour of teaching inorganic chemical tests

In a chemistry lab, you find a bottle labelled magnesium oxide. You assume the powder inside matches the label, but how could you be sure? It’s time to get your hands dirty with some chemical tests.
In this article, we will look at using chemical tests to develop problem-solving skills; the place of chemical tests within chemistry; and using chemical tests as a stimulus to develop student understanding.
Introducing chemical tests
Chemical tests introduce students to analytical chemistry in pre-16 science. Activities such as identifying acids or alkalis using litmus paper or universal indicator serve as a precursor to more advanced chemical tests.
Some students may already be familiar with some chemical tests. For example, pregnancy tests, carbon monoxide alarms and water hardness tests are common in everyday life. We can use everyday chemical tests to help students determine the criteria for a ‘good’ chemical test.
A good chemical test needs to give:
- quick, observable results;
- a definitive and unique response to a particular substance (ion, molecule or atom).
Students will meet chemical tests that naturally divide into three categories:

- flame tests (identifying cations in metal salts);1–3
- gas tests (identifying the presence of hydrogen, oxygen, carbon dioxide, ammonia and chlorine gases); and
- precipitation tests (identifying the presence of metal cations and anions such as halides, carbonates and sulfates).
The Royal Society of Chemistry has published filmed demonstrations of various chemical tests accompanied by a series of quizzes.4 This is a useful resource for introducing and familiarising yourself with the tests.
Developing problem-solving skills
Not all chemical test problems are equally challenging. Using a variety of activities that progressively become more difficult will help develop problem-solving skills in your students. The skills students learn can be built upon in successive activities. For example, the following series of tests increases in challenge for students:
- Identifying acids and alkalis using litmus paper
- Identifying three silver halides: silver chloride, silver bromide and silver iodide
- Identifying metal halides using flame and halide tests
- Identifying unlabelled compounds whose identities are provided5,6
- Identifying compounds without identities provided
The Learn Chemistry resource Five white solids7 is a good example. It requires students to identify unlabelled solids known to be barium chloride, magnesium nitrate, silver nitrate, sodium carbonate and sodium hydrogen sulfate. The strength of this experiment lies in the range of compounds resulting in a number of possible experimental methods to identify the solids. By comparing methods with their peers, students may encounter chemical tests that they otherwise would have overlooked.
When faced with a problem, some students will find it difficult to know where to start. Consider the types of questions that may be useful in helping students along their way without spoon-feeding answers:
- Do any of the compounds have ions in common?
- What chemical test could you use to identify the cation present?
- What chemical test could you use to identify the anion present?
- Is there a single test that will enable me to identify one of the compounds?
Flowcharts
Encourage students to create a flowchart of the chemical tests they plan to use. A flowchart helps students work through their thought process clearly before starting any practical work, as well as being helpful guidance through the experimental procedure. In many cases, the visual flowchart can be a better learning and revision aid than simply a list of chemical tests. A possible flowchart is shown in figure 1.
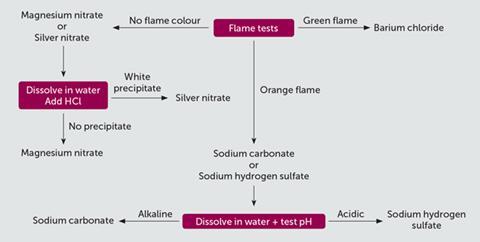
There is no correct way to make a chart like this and students are given the freedom to tackle the problem as they think most appropriate. At the end of the investigation, students can compare their results with their peers and evaluate their method next to methods used by other students.
The following questions can help students evaluate their approach:
- How many separate chemical tests need to be carried out?
- Do the chemical tests require preparation or can they be carried out in a short space of time?
- How many of the chemical tests require extra (expensive) chemicals?
Wider context
Chemical tests bring together many aspects of chemistry and help students make connections between what they can perceive to be unrelated topics. Figure 2 is an attempt to bring together and map some of the connections between chemical tests and other chemistry topics, some of which students meet post-16.
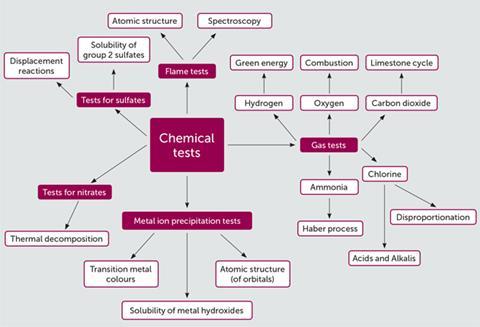
It can be tempting to present the chemical tests in isolation without reference to the wider chemical concepts they relate to. However, in my own experience, a deeper understanding of the chemistry involved in chemical tests helps me to recall the various tests and their applications.
How many students make the connection that limewater turns cloudy because a precipitate of calcium carbonate is formed? How many students understand why barium chloride is used as a test for sulfates and not magnesium chloride? The wider context of the limestone cycle and the solubility of group 2 sulfates helps to make sense of these questions. In this context, chemical tests require students to use the chemistry they already know rather than having to remember more unconnected facts.
Teaching tips
Be ready for subjectivity
Colours look different to different people. Students may disagree if a flame colour is barium or copper green. The halide precipitate may look white or creamy. Consider providing students with reference samples to make it easier.
Head off misconceptions
Students can find it difficult to understand and explain the appearance of a coloured precipitate from two colourless solutions. Vanessa Kind provides some suggestions to address common misconceptions in Beyond Appearances.9
Avoid confusing language
When introducing gas tests, you may want to avoid saying that a gas is ‘released’. This can lead to the misconception that the gas is somehow trapped within the solid or solution, as opposed to the gas being a product of the reaction.
Developing understanding
If chemical tests simply remain a ‘spot the colour change’ exercise, then students may not develop as chemists. As educators, we need to help our students connect the macroscopic observations with the microscopic, using models to develop explanations.8
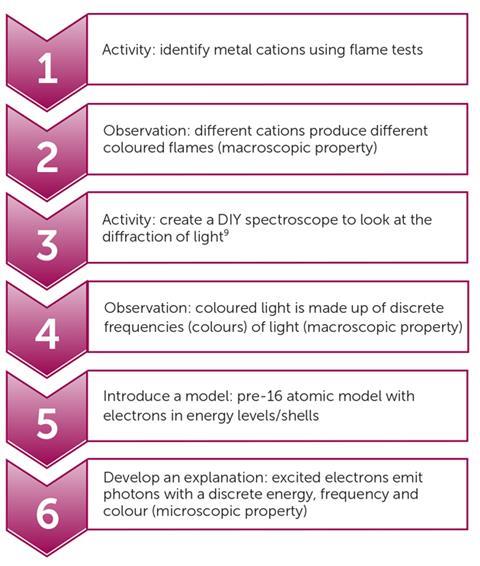
For example, flame tests can be used as a springboard to begin discussing atomic structure and atomic spectroscopy. They serve as a natural bridge to post-16 ideas of UV and IR spectroscopy, tying in with the development of the atomic model as found in many pre-16 specifications. Figure 3 highlights how you can use flame tests as a starting point in investigating atomic structure and quantisation.
With some planning and careful thought, chemical tests can be so much more than lists of colour changes and precipitates that students need to learn. The field of chemical tests provides an opportunity to develop problem-solving and practical skills and reinforce prior learning.
Joe Ogborn is a freelance education consultant, dividing his time between the fields of chemistry and music
More CPD
The Royal Society of Chemistry’s Developing Expertise in Teaching courses are designed to support you throughout your teaching career. Practical work is an integral part of all face-to-face workshops.
Find out more about the CPD for Teachers courses on offer.
References
- Flame tests – spray gun demonstration
- Flame tests – wire method
- Flame tests – wooden splint method
- Qualitative analysis quizzes
- Three white solids
- Education in Chemistry, September 2015, p13, box 3, question 2 (Practical work: a new opportunity)
- Five white solids
- Education in Chemistry, March 2015, p12 (Using models in the classroom)
- V Kind, Beyond appearances: Students’ misconceptions about basic chemical ideas (2nd ed.), p29. Royal Society of Chemistry, 2004 (Beyond appearances)








2 readers' comments