Fundamental topics such as stereochemistry are taught in 2 or 2.5D - the Cambridge Structural Database provides an interactive 3D solution
-
Fundamental 3D topics such as chirality and metal coordination geometry are often taught in 2 or 2.5D
-
It is possible to explore molecular structures and intermolecular interactions using CSD intermolecular-interactions
Chemistry is a 3D subject. Topics such as stereochemistry, chirality, conformation, metal coordination geometry and molecular symmetry are fundamental building blocks of the subject. However, despite the ready availability of interactive molecular graphics software, often at zero cost, structural principles are still frequently taught using 2D images, embellished when required with wedge and dot bonds to provide a 2.5D representation at best. Students must then use their own mental agility to visualise, rotate, invert and perceive symmetry in these chemical objects. In itself, this is fine - every subject needs its shorthand notations - but there is a steep learning curve to reach a point where visual acuity can transform 2D and 2.5D images into full 3D imagery. While 3D graphics and animations using modelled and limited experimental data are extremely valuable, a significant source of 3D structures of varied complexity and broad chemical coverage is needed.
CSD
The Cambridge Structural Database (CSD) has recorded the crystal structures of nearly 600 000 organic and metal-organic molecules.1 A teaching subset2 of some 500 key molecules has been distilled from this vast resource. The subset can be browsed using the WebCSD3 application on the CCDC website. Structures can be visualised and manipulated using a variety of free software packages,
eg Jmol 4 or Mercury.5
Teaching with CSD structures
We can show how some basic chemical concepts can be taught using CSD structures. All of these illustrations have been prepared using the CCDC's Mercury program. Although only static images can be presented here, the images are fully interactive online for the student and instructor. The program also allows you to the measure molecular geometry such as bond lengths, valence angles and torsion angles, by clicking on individual atoms.
Conformational analysis and stereochemistry
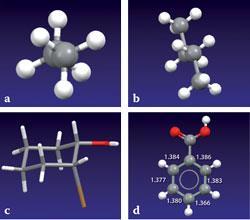
The simple alkanes provide examples of eclipsed (high energy) and staggered (minimum energy) conformations about their C-C single bonds, which is the standard student introduction to conformational analysis. With the CSD, students can view the staggered ethane conformation along the C-C bond (fig 1a). They can then view n-butane, where each central carbon now has one methyl substituent instead of a hydrogen atom, and examine the mutual disposition of these larger groups. Fig 1b shows that the two methyl groups are arranged as far apart as possible within the expected staggered arrangement. The preferred chair conformation of cyclohexane is illustrated in 2-bromo-cyclohexanol (fig 1c) which has equatorial hydroxy and axial bromo substituents. The coplanarity of the carboxylic acid substituent with the planar benzene ring in benzoic acid is shown in fig 1d, together with the aromatic ring bond lengths determined in the experiment.
Chirality
The chirality assignments for naturally-occurring L-alanine (fig 2a) and its artificial D-enantiomer (fig 2b) are the topic of one of the teaching modules on the CCDC website. Both enantiomers are shown viewed along the C-H bond (H pointing away from the viewer) at the stereogenic centre, H being the lowest priority substituent in the Cahn-Ingold-Prelog (CIP) formalism. Students can then identify the CIP priority order of the other substituents:
NH3+ > COO- > CH3
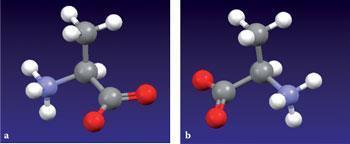
and determine whether the circular pathway from first to third priority is anticlockwise (S, as in the natural L-form) or clockwise (R, as in the artificial D-form). They can also interact with the images (eg rotate, translate, etc) and reassure themselves that the R- and S-enantiomers are not superimposable.
Molecular structure and shape
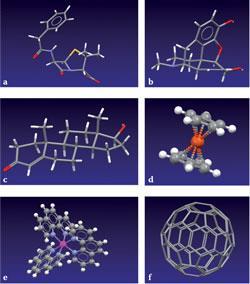
Crystallography is the preferred method for structure determination for both novel and common molecules: while common molecules abound, the unusual is commonplace! This coverage makes the CSD ideal for exploring the exciting diversity of chemical structure in all its aspects, and six varied examples that can encourage student exploration and understanding are shown in fig 3: (a) benzylpenicillin G, (b) morphine, (c) testosterone, (d) ferrocene, (e) the octahedral coordination of bidentate ligands in tris(1,10-phenathroline)-osmium, and (f) C60-fullerene.
Given over half a million structures to choose from, this selection is obviously subjective, and local subsets can easily be constructed to exemplify various aspects of any chemistry course, eg aromaticity, ring strain, absolute configuration, etc, or tailored to any special chemical area, eg amino-acids, pharmaceuticals, metal coordination geometries, and so on.
Intermolecular interactions
Understanding non-covalent interactions, especially hydrogen bonding, is vitally important to chemistry and the life sciences students. Non-covalent interactions of all types, whether mediated by hydrogen or not, are fundamental to the burgeoning field of supramolecular chemistry. In the life sciences, hydrogen bonds are responsible for the structural organisation of DNA, RNA and proteins. They are also crucial in protein-ligand interactions that are responsible for the activity of pharmaceuticals and agrochemicals.
Crystallography makes a major contribution to our knowledge of intermolecular interactions, particularly in terms of their geometry and directionality. A crystal structure comprises an infinite array of molecules and/or ions having an extended structure that is stabilised by hydrogen bonds and other non-bonded interactions. Because atomic coordinates in small molecule crystal structures are determined experimentally at high resolution, the crystallographic technique provides direct and accurate observations of the geometrical and spatial characteristics of individual interactions. Extended structures can be generated, viewed and manipulated in both Jmol and Mercury, and their geometrical characteristics can be measured interactively by atom-clicking.
Crystallography, the CCDC and the Cambridge Structural Database
Crystal structures:
- are determined from the intensities of x-rays or neutrons diffracted by the crystal lattice
- are described by a set of 3D atomic coordinates with respect to the unit cell of each crystal
- provide very precise geometrical data for inorganics and small molecules

Crystallography has been involved in 25 Nobel prize awards.
The Cambridge Crystallographic Data Centre (CCDC)
CCDC was founded in 1965 at the University of Cambridge and is now a fully independent self-financing registered charity for the promotion of crystallography and chemistry in all their aspects.
Cambridge Structural Database System (CSDS) includes:
- all published crystallographic studies of organic and metal-organic small molecules ('small' means up to 1000 atoms including hydrogens)
- associated software tools for search, retrieval, visualisation and analysis of data details of 567 252 crystal structures (June 2011).
- details of 567 252 crystal structures (June 2011).
Each CSD structural entry contains:
- atomic coordinates and other crystal data
- 2D chemical diagram
- bibliographic and physicochemical information
- all data are checked and evaluated
Accessing the CSD for teaching
- The CCDC website
- The CSD teaching subset and other teaching resources can be accessed and browsed online by following the links on CCDC website.
- The full CSD system is available for an annual fee, but with significant discounts for teaching applications. To find out more and discuss teaching applications, contact the CCDC teaching team
Some examples
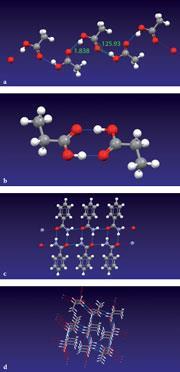
Four Mercury examples are shown (fig 4a) the H-bonded O-H...O chains (with geometry) that are present in the structure of ethanoic acid, (b) the more common H-bonded dimers formed by the O-H...O in propanoic acid, and (c) the N-H...O bonds that form two linked ring motifs which generate 2D molecular layers in the structure of benzamide. Students might then like to use Mercury to build and explore the 3D H-bonded network formed by L-alanine (fig 4d), and learn about the complex H-bonding possibilities that are open to simple amino acids. This will help them to appreciate the even more complex H-bonded systems observed in peptides and proteins.
CSD-based teaching modules
The teaching area of the CCDC website also contains a series of modules that use both the teaching subset of the CSD and the complete CSD itself to address specific chemical topics. These include (teaching subset)6: aromaticity, ring strain and conformation, valence shell electron pair repulsion, and hapticity, as well as (full CSD)7: mean molecular dimensions, halonium ions as reaction intermediates, metal-carbonyl back bonding, and geometrical interconversions in four-coordinate metal complexes. This set of modules is now being extended to include, among others, studies of reaction pathways and studies of hydrogen bonding and other intermolecular interactions.8
Get involved
Most of the published teaching modules address topics commonly found in undergraduate chemistry courses and many arise from the CCDC's collaboration with Greg Ferrence at Illinois State University, US. Several modules can be used within an independent learning environment. The CCDC would be delighted to hear from readers who have suggestions for extending the range of these modules. Relatively little work has so far addressed the use of CSD information in the curricula of secondary (high) school courses.
The CCDC is now involved in school outreach activities through a collaboration with Peter Hoare at the University of Newcastle, UK. We are particularly interested to hear from school chemistry specialists who would like to discuss this potential and help develop effective mechanisms for delivering CSD-based learning tools at school level. The potential for using CSD information within a program of independent learning is a special interest.
Frank Allen is an Emeritus Research Fellow at CCDC. Susan Henderson and Gary Battle run the Centre's educational outreach activities.
References
- F H Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, B58 , 380 (DOI: 10.1107/S0108768102003890).
- G M Battle et al, J. Chem. Educ., 2010, 87, 809 (DOI: 10.1021/ed100256k)
- I R Thomas et al, J. Appl. Crystallogr., 2010, 43, 362 (DOI: 10.1107/S0021889810000452)
- R M Hanson, J. Appl. Crystallogr., 2010, 43, 1250 (DOI: 10.1107/S0021889810030256)
- C F Macrae et al, J. Appl. Crystallogr., 2008, 41, 466 (DOI:10.1107/S0021889807067908)
- G M Battle et al, J. Chem. Educ., 2010,87, 813 (DOI: 10.1021/ed100257t)
- G M Battle et al, J.Chem. Educ., submitted
- G M Battle et al, J. Appl. Crystallogr., 2010, 43, 1208 (DOI: 10.1107/S0021889810024155)









No comments yet