Thermoelectric materials can be assembled into mechanical structures which can transform heat to electrical energy. They can be used for heat harvesting and refrigeration.
- Thermoelectric materials can be used for heat harvesting and refrigeration
- Efficiencies lie in the development of new composite nanomaterials
Thermoelectricity
Thermoelectricity is the conversion between heat and electricity. All materials exhibit thermoelectric effects but the name 'thermoelectric materials' is used to describe the materials that are good at converting heat to electricity.
Uses for thermoelectric materials
Thermoelectric materials are used in niche cooling applications, for example to maintain very stable temperatures in lasers and optical detectors, and they are often found in office water coolers. They are also used in space exploration to convert heat from a radioactive material into electricity.
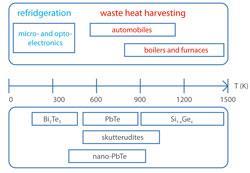
The current focus on energy sustainability and stricter legislation on the emissions of CO2 imposed on automobile manufacturers has sparked a great deal of interest in these fascinating materials.
Only about a third of the fuel energy is converted into mechanical energy in an internal combustion engine with the remainder lost as heat. A thermoelectric generator harvests waste heat from the exhaust gases, which are at a temperature of 300-500ºC, and turns this into electricity. State of the art modules generate about 1 kW, which can be used to power the electrical equipment in the car. This allows for a smaller alternator, which reduces the roll friction, leading to an increase in fuel efficiency and reduced CO2 emissions.

How does it work?
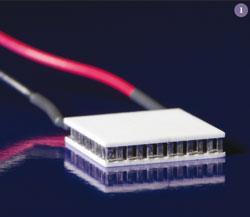
Thermoelectric modules, like the one shown, (fig 1) consist of p- and n-type semiconducting 'legs' sandwiched between electrically insulating plates. All that is needed is a temperature difference between the top and bottom plates, and electricity will flow through an external circuit.1 The efficiency of the thermoelectric module is largely determined by the properties of the p- and n-type legs but also by the temperature difference with larger gradients being preferred.
Fig 2 shows diagrams of thermoelectric couples. On the left (fig 2a) you can see how the device is used in power generation mode. The temperature difference between top and bottom drives the electrons and holes (missing electrons) away from the hot side. This leads to an imbalance in charge between the hot and cold sides. In other words there is a voltage difference, just like a battery, that can be used to do electrical work, eg powering the radio in your car.
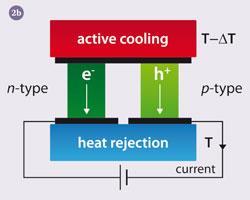
The reverse is also possible, which you can see in fig 2b. Here a current is forced through the device. The electrons and holes are moved away from the junction dragging along heat, which leads to the cooling of the top plate. Reversal of the current direction leads to the warming up of the top plate.
Thermoelectricity
So why is thermoelectricity not used more widely? The reason is that the coupling between the electrical and heat currents is weak in most materials, and the overall energy conversion efficiency is therefore very low. You need a lot of heat to generate a little electricity. Is that the end of it? Well no, researchers are working hard to discover new p- and n-type semiconductors, which can do this more efficiently.
Current research
The thermoelectric efficiency of a material is quantified by a figure of merit (z), which contains three physical quantities:
- Seebeck coefficient (S)
- electrical conductivity (σ)
- thermal conductivity (k)
The Seebeck coefficient tells us how many volts per degree temperature difference are generated. The electrical conductivity determines how well a material conducts electricity, and the thermal conductivity is a measure of how well heat is conducted.
The first two quantities need to be as big as possible, while the last one needs to be small to be able to maintain the temperature difference between the hot and cold side. The problem is that all three quantities are related, and cannot be optimised independently.
It turns out that semiconductors provide the best compromise with commercially used refrigeration materials, such as Bi2 Te3 having figures of merit:
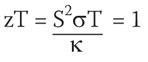
Here T is the absolute temperature, and the other parameters were defined above. There is no fundamental upper limit on zT but for decades it was limited to values around one. The bigger the value of zT is, the greater the energy conversion efficiency of the material.
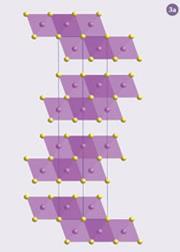
The crystal structure of Bi2 Te3 contains sheets separated by gaps in which there is no covalent bonding (fig 3a). These gaps are one of the factors that give this material a low thermal conductivity. However, the potential for chemical modifications and so improvements in the figure of merit, in a structure containing only two elements is limited.
New methodologies
About 15 years ago, researchers started to develop new methodologies to tackle the apparent zT = 1 limit on the thermoelectric performance. The background to all these approaches is the decoupling of the electronic and thermal transport. In other words, how can we make a material with a simultaneously large Seebeck voltage, a large electronic conductivity and low thermal conductivity? Something that is impossible for traditional materials such as Bi2 Te3.
The first paradigm was that of the phonon glass electron crystal. This approach combines the good electronic properties of a crystal with the low thermal conductivity of a glass. A crystal (eg Bi2 Te3) (fig 3a) has a perfect ordered arrangement of atoms, while in a glass the nearest neighbours have a well defined arrangement but there is no ordering of atoms on longer length scales. This lack of long range ordering means that glasses have the lowest thermal conductivities of all known materials. Unfortunately, they have poor electronic properties.
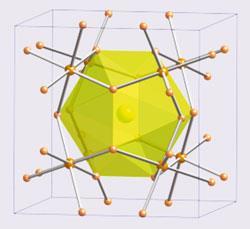
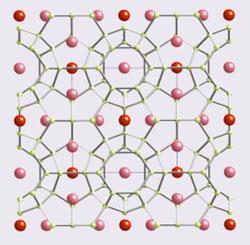
Crystals, in contrast, support good electronic conduction but often have large thermal conductivities. So, by creating a material with both glass and crystal properties, outstanding thermoelectric performance can be achieved. Of course, simple materials, such as Bi2Te3, cannot be both glass and crystal. However, chemically and structurally complex materials have been found to contain both properties as illustrated by the skutterudites and the clathrates (fig 3b,c). In these materials, a covalent network (the electron crystal) is optimised to provide good electronic properties, while the lattice conductivity is kept low by the heavy loosely bound 'rattling' ions (the phonon glass). This leads to substantial increases in the figure of merit with values up to zT = 1.5 being achieved.
Another step forward
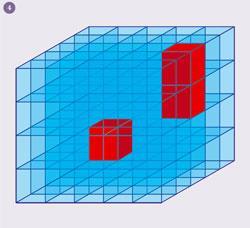
The second big step forward came from a theoretical prediction that large Seebeck coefficients could be achieved in nanostructured materials. These are composite materials where nanometre regions of material A are embedded in material B (fig 4). The increase in the Seebeck coefficient comes from changes in the electronic structure when the dimensionality of a material is reduced. This concept was proven in carefully grown quantum dot thin-films but has been found difficult to translate to bulk materials. However, fortunately, the large number of interfaces in nanostructured materials are very efficient at reducing the lattice thermal conductivity. This happens because lattice vibrations behave like waves in a solid. The propagation of waves with wavelengths shorter than the size of the nanodomains are not affected but waves of similar length are scattered very effectively. It is this effect that leads to a large reduction in thermal conductivity.
Lead telluride
The best studied bulk nanocomposite material is based on lead telluride (PbTe), one of the traditional thermoelectric materials, which spontaneously forms a nanocomposite. For example, this happens when PbTe is mixed with small amounts of AgSbTe2 and cooled from a melt. Materials of this kind have a thermal conductivity close to the glass limit and a figure of merit approaching two. This seminal discovery3 led to a huge amount of interest in nanostructured bulk materials based on the already known good thermoelectrics. For example, the figure of merit of Bi2 Te3 has been improved by 50% in nanostructured samples.
Outlook
The figure of merit has almost doubled in the past 15 years from one to almost two. This means that thermoelectric power generation could become 10-20% efficient if these new materials can be successfully used in devices. However, currently most of the top performing thermoelectric materials rely on scarce elements, such as tellurium. For this reason alternatives, even if lower performing, are of interest. Among these are traditional solid state materials, such as metal oxides and silicides but other classes of materials including conducting polymers are also attracting interest.
A recent paper reported the fabrication of plastic thermoelectric modules with a few percent energy conversion efficiencies from a small temperature difference.4 Another interesting approach is the harvesting of solar heat, which is not converted to electricity by photovoltaic cells. The sun emits about 40% of its energy in the infrared region. A laboratory prototype device using a simple concentrator and nanostructured Bi2 Te3 enabled a 5% conversion efficiency, which compares to about 15-20% for solar cells.5
Recent developments have given a toolbox of approaches to increase the thermoelectric performance. Now this has to be applied to cheaper materials that will enable the large scale use of this technology. If this materials research challenge can be met, it seems certain that thermoelectric materials will play an important part in a sustainable energy future.
Jan-Willem Bos is a lecturer at the Department of Chemistry, School of Engineering and Physical Sciences at Heriot-Watt University, Edinburgh.
References
- MRS Bull., 2006, 31, issue 3 www.mrs.org/bulletin see also the website on thermoelectrics at http://thermoelectrics.caltech.edu/ (no longer available)
- P Vaqueiro and A V Powell, J. Mater. Chem., 2010, 20, 9577(DOI: 10.1039/C0JM01193B)
- K F Hsu et al, Science, 2004, 303, 818 (DOI: 10.1126/science.1092963)
- O Bubobna et al, Nat. Mater., 2011, 10, 429 (DOI: 10.1038/nmat3012)
- D Kraemer et al, Nat. Mater., 2011, 10, 532 (DOI: 10.1038/nmat3013)
- J He et al, J. Mater. Res., 2011, 26, 1762 (DOI: 10.1557/jmr.2011.108)









No comments yet