Neil Withers investigates a compound with some unusual and unexpected behaviour
When you heat things up, they generally get bigger. That’s what I learnt at school, and for most things it holds true. But not all. There are some materials that actually shrink as they get hotter, and zirconium tungstate, ZrW2O8, is one of the most studied and best understood.
To continue my nostalgia for my school science classroom, let’s think back to how we were taught about thermal expansion. I remember my teacher telling us to imagine we were atoms. If we had more energy, and were running around the room instead of sitting quietly, we’d start bumping into things and would need more space. And that’s roughly how it is for atoms: give them more heat energy and they vibrate more, needing more room between them.
Structure is key
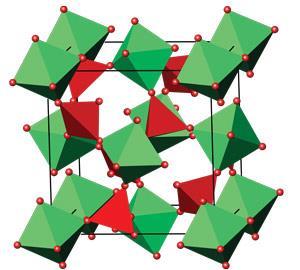
The key to zirconium tungstate’s counterintuitive property is bound up in its structure, as with so many things in chemistry. And its structure is best described in terms of zirconium oxide octahedra and tungsten oxide tetrahedra. In both cases, the metal atom is at the centre with oxygen atoms at the points. The zirconium oxide octahedra are in a face-centred cubic arrangement – not touching each other, but instead sharing corners with the tungsten oxide tetrahedra. Another way to think about it is as the common sodium chloride or rock salt structure, with zirconium oxide octahedra in place of the sodium and tungsten oxide tetrahedra replacing the chloride.
These hand-holding polyhedra are what gives zirconium tungstate its negative thermal expansion. One of its main vibrational modes is for individual octahedra and tetrahedra to wobble back and forth with the oxygen atoms moving a lot while the metal atoms move less. This guitar-string-style vibration slightly pulls the metal atoms together, reducing the overall size of the material. You can do it yourself: bunch your hands into fists, then put them together in front of your chest. Move them up and down enough and you’ll notice that your elbows move closer together.
With all this talk of things shrinking on heating (and vice versa), you may now be thinking about the best-known example of a substance that enlarges when cooled: water. But this effect only happens during the transition between its liquid and solid phases. Zirconium tungstate, on the other hand, contracts all the way from 2 Kelvin up to 1400.
An exotic creature
Besides its undoubted interest as a curious and interesting phenomenon, what use is negative thermal expansion? Well, at the moment, sadly not much. Regular old thermal expansion causes problems in a number of places, from expanding metal train rails to ceramic ovenware shattering when mistreated. Creating ceramics with some shrinking and some expanding components could result in materials that neither expand nor contract when heated – so they would be impervious to thermal shock.
Although cracking and shattering kitchenware is annoying, there’s another place where thermal shock can be more painful: your teeth. Rapidly changing from a hot to a cold environment (or vice versa) can often happen when eating and drinking. And if your fillings expand and contract at a different rate to your teeth, they are more likely to be painful – or even fall out. So being able to make materials with a tailored expansion that exactly matches that of your teeth could save many people from aching teeth.
Although pain-free fillings would be desirable, these applications are perhaps a little underwhelming for such an unusual physical property – I prefer to think of ZrW2O8 and its fellow negative thermal expansion materials as wonderful and exotic creatures in the chemical zoo. They show behaviour very few other materials do, and it’s behaviour you never would expect.









No comments yet