This chapter discusses the nature of chemical concepts and how such concepts are learnt
The problem of clearly defining key chemical concepts such as ’element’ and ‘molecule’ is explored, and the implications for teaching are considered.
What are chemical concepts - and why are they hard to learn?
The term ’concept’ is used a lot when talking about learning, but it is one of those words - perhaps like ’molecule’ -which, although we seem to know what we mean by it, is not so easy to define precisely.
A concept is just a way of breaking up the world into bits that we can recognise, and think about. Some of our concepts - so called identity concepts - refer to specifics such as your particular school or college, or particular people.
People the world over tend to categorise certain things in the same ways. For example we all use categories such as ‘tree’ without having to think about how such terms are defined. These concepts seem to be largely intuitive.
In science, we are largely dealing with rule-governed rather than natural concepts. In other words, we often define a new concept. The natural category meanings are usually learnt before the scientific ones, and are very commonly used in everyday life, therefore it is difficult to expect students to give them up.
In chemistry these problems may seen less extreme than in biology, or physics, where most of the key concepts are blighted by having labels which are also used in more vague everyday senses, but we may still find students using words like ’natural’, ‘synthetic’, ‘metal’ ’pure’ and ’substance’ in ways which do not match the technical meanings.
Forming concepts - noticing similarities and differences
In one sense a person can be said to have acquired a concept once they are able to identify examples, and distinguish them from non-examples.
For example, consider the two figures below:
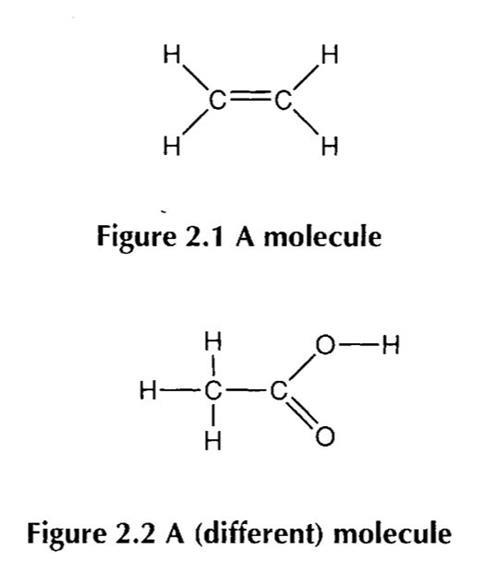
A student may recognise that they are both examples of molecules, and perhaps that they are both molecules of substances considered organic. The student might identify that both compounds include a double bond. Perhaps the student could identify the first as an example of a hydrocarbon, and further as an alkene and even as ethene; and the second as a carboxylic acid, and perhaps as ethanoic acid.
Making these classifications requires the students to recognise certain attributes of the species represented as pertinent to these various concepts.
Providing clear definitions
Many chemical concepts are only ‘simple’ once they are understood as part of a much wider network of ideas.
So consider the following definition:
An alcohol is a compound with an -OH group.
This definition would include ethanol etc, but could also apply to ethanoic acid, and even sulfuric acid -which are not considered alcohols. So we would need to add something about the alcohol being an organic compound and the -OH group not being part of a larger -COOH group.
If we defined an alkene as having an empirical formula of CnH2n, then we would need to add the proviso that it was not a cyclic molecule.
There is a real issue here of finding the optimum level of simplification, of balancing the need to keep things simple now, and providing learners with ideas which we are not going to expect them to alter later. Learners do not always find it easy to adjust meanings for technical words once they are acquired. It could be argued that science is largely about culturally agreed models that scientists use to make sense of the world.
Defining key concepts in chemistry
It is useful to illustrate the problems associated with defining chemical concepts.
Chemical and physical changes: chemistry is largely concerned with the properties and reactions of substances. Properties are often classed as physical (colour, melting temperature …) and chemical (which substances are reacted with, under what conditions, to give which products). Reactions, chemical changes, are a central part of the subject. An understanding and application of the distinction between physical and chemical changes is often expected of students at lower secondary level.
It is reasonable to suggest that;
- It is difficult to form a detailed appreciation of chemistry without a fair understanding of what is meant by these terms.
- Practising chemists, and chemistry teachers, would have a clear understanding of what these ideas mean.
Notes
For the full version of this chapter, see downloads below.
Downloads
Concepts in chemistry
PDF, Size 0.63 mb
Websites
Additional information
These PDFs have been taken from the popular book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 1, by Keith Taber.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
 Currently
reading
Currently
reading
Concepts in chemistry
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36





























































































No comments yet