Many students believe that chemical reactions occur to enable atoms to obtain full outer electron shells, or ‘octets’
This belief is so strong that they tend to offer this explanation even when they have been taught more appropriate ideas.
Why do hydrogen and fluorine react?
Hydrogen reacts with fluorine to give hydrogen fluoride. The equation for this reaction is:
H2(g) + F2(g) →2HF(g)
The word equation is:
hydrogen + fluorine →hydrogen fluoride
Look at the following diagrams:
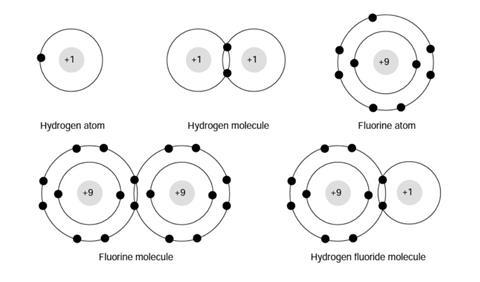
In your own words, explain why you think hydrogen reacts with fluorine.
Notes
For the full version of this chapter, see downloads below.
Downloads
Hydrogen flouride
PDF, Size 0.24 mb
Websites
Additional information
These resources have been taken from the book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 2, by Keith Taber.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
 Currently
reading
Currently
reading
Hydrogen fluoride
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36





























































































No comments yet