This short written activity is designed to show the power of the Periodic Table in predicting patterns
In the activity fluorides of the elements are used because fluorine reacts with most elements. Download editable and pdf versions of the worksheet with answers below.
Fluorine is a very reactive element and reacts with the vast majority of other elements to form fluorides. It is so reactive that it reacts with some of the elements in Group 0 – the so called ‘inert’ or ‘noble’ gases. The table below shows the formulas of some of the compounds formed between the elements and fluorine.
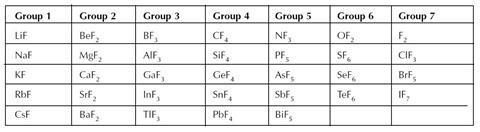
Questions
- What do you notice about the formulas of the fluorides of Groups 1, 2, 3 and 4?
- What do you notice about the formulas of the fluorides of Group 7?
- What do you notice about the formulas of the fluorides of the first horizontal period (row)?
- If bromine formed compounds with the elements shown in the table, what would you expect their formulas to be? Predict the patterns you would expect to see.
Answers
- The fluorides of the elements in Groups 1, 2, 3 and 4 have formulas in which the number of fluorine atoms matches the group number.
- The formulas of the fluorides of Group 7 show that the number of fluorine atoms per molecule increases as you descend the group. You may want to have an explanation ready as to why such non-metals react – fluorine is the most reactive element. The increase in the number of fluorine atoms bonded to the central atom can be explained in terms of the increasing size of the central atom.
- The fluorides of the elements in the first horizontal row have formulas in which the number of fluorine atoms per molecule is the group number or 8 minus the group number.
- Bromine would be expected to form compounds with similar formulas to those of the fluorides.
Downloads
Patterns in formulas
Handout | PDF, Size 0.21 mbPatterns in formulas
Editable handout | Word, Size 64.04 kb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
 Currently
reading
Currently
reading
Patterns in formulas of compounds
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35























































































No comments yet