Explore the polymerisation of lactic acid to polylactic acid, a useful commercial plastic
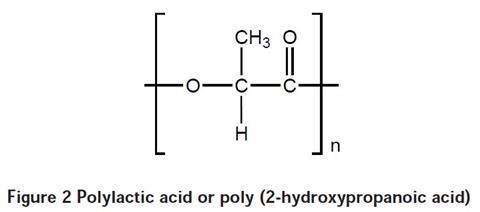
Lactic acid (2-hydroxypropanoic acid) can be obtained by fermenting glucose or maltose or can be extracted from milk. It is used as the starting point for the production of polylactic acid, also known as poly (2-hydroxypropanoic acid), which can also be made from petrochemicals. Polylactic acid is a condensation polymer – a molecule of water is produced for every link made in the chain. This is not specified in the students’ notes, but you might wish to draw their attention to it.
When the water is removed, an oligomer made of 10–30 lactic acid units is formed. An oligomer is essentially a short length of polymer. The production of true polylactic acid involves catalytic depolymerisation of the oligomer to a lactide intermediate followed by polymerisation of the lactide to high molecular weight polylactic acid. In the experiment described here only the oligomer is made.
The product therefore has different properties from the polylactic acid used in packaging. It may be useful to discuss with students how the different properties are related to the chain length of the polymer molecules.
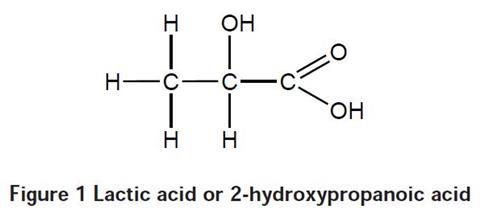
During polymerisation, the OH on the acid group (CO2H) group of the monomer reacts with the OH group on the second carbon atom of another monomer. This is an esterification reaction and involves the loss of water. The resulting polymer is shown in Figure 2.
Equipment required
For each group of students:
- Test-tube
- Test-tube holders
- Bunsen burner and heat proof mat
- Anti-bumping granules
- Lactic acid (2-hydroxypropanoic acid) (Irritant)
- Hydrochloric acid 2 mol dm–3 (Irritant)
- Petri dish or white tile
- Eye protection.
Health, safety and technical notes
- Wear eye protection
- Read our standard health and safety guidance
- The boiling point of lactic acid is 122 °C so when students pour it out it will be very hot. Warn students to take care.
Notes
- Lactic acid is very viscous so do not attempt to get students to use measuring cylinders.
- A 1/5 full test-tube works fine and measuring the lactic acid in this way saves making several measuring cylinders very sticky.
- The product is very sticky, so discourage students from touching it. They should use a glass rod or a wooden splint instead of their fingers
What to do
- Fill a test-tube 1/5 full with lactic acid.
- Add 5 drops of hydrochloric acid and two anti-bumping granules.
- Put the test-tube holders around the top of the test-tube and begin to heat the tube. Be careful not to point the open end of the test-tube at anyone in the room – try to point it towards a wall.
- Keep the mixture gently boiling and stir or gently shake the tube occasionally to mix the contents.
- After about 10 or 15 minutes the mixture will begin to go a yellowish colour. Leave it for another minute or two and then quickly pour the contents of the tube out onto either a petri dish or a white tile.
- Leave the mixture to cool.
Questions
- If you have successfully polymerised the lactic acid, what will have happened to the size of the molecules?
- Look at the lactic acid you started with and your product. Describe the differences in properties between them (be careful – your product will be very sticky).
- Try to explain the change in properties in terms of the size of the molecules in the materials.
Answers
- The molecules will have got bigger because during polymerisation, the small molecules join together in chains.
- The lactic acid is quite viscous, but the product is a solid (or a very viscous liquid). The product does not run off a petri dish or fall off if you turn it upside down. Note: You can also test for water solubility – the starting material is water soluble but the product is not.
- As the molecules get larger, the intermolecular forces holding them together also get larger. This causes the material to become stickier and more viscous. (Larger molecules also tend to move around less so materials with large molecules are often solids.)
Downloads
Polylactic acid
PDF, Size 0.39 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
 Currently
reading
Currently
reading
Polylactic acid
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35























































































No comments yet