Find yourself on the spectroscopy spectrum
Three activities that could be used to teach spectroscopy are presented within this resource. A good introduction to spectroscopy is to do the Cold light practical or to demonstrate that many objects absorb UV light and then emit light of a different wavelength/frequency. Explain that the same thing happens with infrared radiation (and many other types of electromagnetic radiation) except that we cannot see it. Instead, a detector is used to read which wavelengths of infrared are being absorbed (or transmitted). Every substance has its own unique infrared (IR) spectrum.
A spectrum obtained during an experiment can be matched against a data bank of sample spectra to identify a substance. It is also possible to interpret certain individual peaks on the spectrum using a data book/sheet. When IR radiation interacts with matter and is absorbed, it causes the bonds in the substance to vibrate.
They can either bend or stretch – this is the basis of the activity Chemical aerobics, which is another good introduction to the topic of spectroscopy. Two problem-solving activities relating to IR spectroscopy are given in the student sheet entitled Spectroscopy.
Chemical aerobics
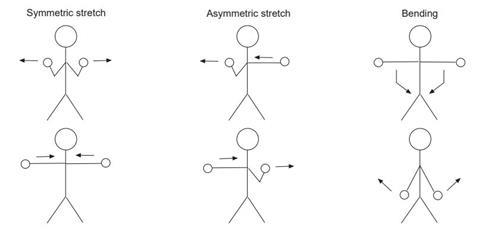
When infrared radiation interacts with a substance, certain wavelengths cause particular bonds in that substance to vibrate. Only certain wavelengths will do this and each different type of bend or stretch of a bond will require a different wavelength of radiation. Get the class to stand up and pretend that they are each a water molecule. Their heads are the oxygen atoms, their hands the hydrogen atoms and their arms the bonds.
If you want to make this really clear, you can make large labels for the heads and hands. Ask the students to try to work out which bends or stretches are possible. They should be able to work out that there are three possible kinds of vibration, although they almost certainly will not get the names for them. The three vibrations are:
- symmetric stretch (both hands moving back and forth away from the body together and both arms bending together)
- asymmetric stretch (one hand moves forward as the other moves back towards the body, or one arm bending as the other straightens)
- bending (hands moving towards each other in front of the body and back again with arms staying straight).
If you wish to make this exercise more fun, you could have some music clips prepared on a tape or CD. Assign a different singer/group/song to each movement. When the students hear the singer they have to make the correct move – if there is a singer with no movement assigned then they stay still. You can be as cheesy or up-to-date as you like but the singers do need to be well known by the vast majority of the class and the clips need to be short.
Spectroscopy
Many substances absorb light at one wavelength and emit it at another. We make use of this in many ways, for example in glow-in-the-dark stickers. Compounds can also absorb and emit radiation that we cannot see, such as infrared, microwave and ultraviolet radiation. Finding out which radiation is absorbed and emitted can tell us a lot of useful information about a compound.
Put this in context
Find out more about museum scientist Lucia, who uses her chemistry knowledge to analyse museum objects.
This branch of chemistry is called ‘spectroscopy’. It is used in a very wide variety of applications, from helping chemists to work out the structure of a new molecule they have made to testing for drugs, forensics testing and quality control. Spectroscopy can even be used to help museum conservators assess what damage has occurred to an object. Infrared (IR) spectroscopy involves firing infrared radiation at a substance and measuring the radiation that is absorbed by the molecules. It is particularly useful for studying organic (carboncontaining) chemicals.
The spectra that result look quite complex – each one is a line with a series of ‘bumps’ called peaks at the particular wavelengths where radiation is absorbed by the sample. However, it is often not necessary to decide what each peak represents in order to get useful information from a spectrum.
Different bonds cause peaks (we call them peaks, even though they are actually troughs) in particular places in a spectrum.
Downloads
Spectroscopy
PDF, Size 0.95 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
 Currently
reading
Currently
reading
Spectroscopy























































































No comments yet