Use marble, water, oil and washing-up liquid to observe the behaviour at the surface of substances
The aim of this activity is to introduce students to the idea that the surface of a substance may behave differently from the bulk material. This is achieved by observing two familiar experiments closely.
It is worth carrying out both experiments to get the point across.
Practical activity 1 – equipment
- Hydrochloric acid – 2 mol dm–3 works well
- Marble chips – at least 7 mm in diameter
- Calcium carbonate powder – label as ‘crushed marble’
- Small beakers or boiling tubes
- Measuring cylinders
- Timers.
Practical activity 2 – equipment
- Oil – corn oil works well as it is slightly darker than sunflower oil
- Detergent – good quality washing-up liquid
- Blue food colouring – optional, but it does make the microscopy easier.
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear eye protection
- Hydrochloric acid is an irritant, see CLEAPSS Hazcard HC047a
Practical activity 1 diagram
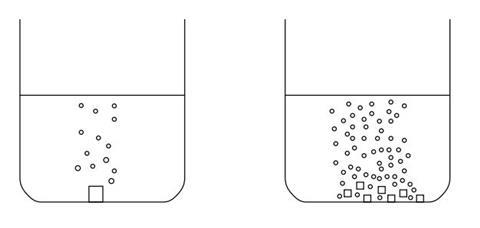
What to do
- Weigh a marble chip.
- Weigh out the same mass of crushed marble onto a piece of paper.
- Measure out 20 cm3 hydrochloric acid 2 mol dm–3 (Irritant) into two small beakers.
- Add the powder to the beaker.
- Time how long it takes to finish reacting.
- Watch carefully what is happening to the powder in the beaker.
- Repeat with the lump of marble.
Practical activity 2
In this activity you are going to investigate what happens when oil, water and detergent are mixed.
What to do
- Take two boiling tubes.
- Put about 25 cm3 water and 25 cm3 oil into each.
- Put a bung into one of the tubes and shake it.
- Leave it for a few minutes.
- Add a few drops of detergent (washing up liquid) to the other boiling tube.
- Observe what happens at the boundary between the oil and water.
- Watch carefully for a couple of minutes, then put a bung in the boiling tube, shake it and leave it for a few minutes.
- Put a few drops of your mixture onto a microscope slide and look at it at low and medium power.
- If you have difficulty focussing the microscope, add a drop of blue food colouring to your boiling tube mixture, shake it and try again.
- Draw and label what you can see under the microscope.
Questions
- Which will react faster – the lump of marble or the powder? Try to explain why by thinking about particles. Draw a diagram to help you explain
- Did the results match your prediction? If not, explain your observations in terms of particles.
- Did all the lump of marble react with the acid at the same time? Which part of the marble chip reacted with the acid?
- What would happen to the rate of the reaction if you made the pieces of marble in the powder even smaller?
- Where are the oil and the water touching each other?
- Where are the oil and the water touching each other now that you have added detergent?
- What has happened to the amount of oil touching the water?
- What has formed at the surface where the water touches the air?
- Do the particles at the surface of a substance behave in the same way as the ones in the middle?
Answers
- The marble powder will react faster. This is because the acid can only react with the atoms/particles on the surface of the marble as particles from the two substances have to collide in order to react. The powdered marble has more atoms/particles on the surface overall and so more reactions can take place at the same time.
- The results should back up the prediction, otherwise an explanation similar to that given in the answer to question 1 should be included here.
- Students should be able to observe that gas is only given off from the surface of the large lump of marble – they should therefore be able to deduce that only the surface reacts with the acid.
- As the pieces get smaller, the reaction should get faster as more atoms/particles are at the surface and available to react with the acid.
- The water and oil are touching each other only at the surface between them.
- Without detergent, the oil and water do not mix at all to start with – they form two completely separate layers. When the boiling tube is shaken, they mix but quickly separate back out into two layers. When the detergent is added the oil and water start to mix at the boundary between the layers. Small balls of oil sink through the water, but most of the oil remains separate from the water. When the detergent mixture is shaken, the liquids mix and remain mixed.
- The amount of oil touching the water has increased.
- Foam has formed at the surface where water touches air.
- Particles at the surface do not behave the same as the ones in the middle.
Downloads
The surfaces of substances
PDF, Size 0.21 mb
Additional information
This resource is a part of our Inspirational chemistry collection.
Inspirational chemistry book

A collection of resources, aligned with GCSE bodies, to support learners in England, Wales, and N Ireland.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
 Currently
reading
Currently
reading
The surfaces of substances
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35























































































No comments yet