Energise your students with these classroom tips and ideas when teaching this tricky topic

Why does zinc react with copper sulfate producing zinc sulfate and copper? Why do diamonds ‘last forever’? To answer these questions, we need to delve into the central but abstract world of energetics.
Energy is a tricky concept, and one that all students must get to grips with across the sciences. Unfortunately, chemists, physicists and biologists sometimes talk about energy in different ways, and this can lead to misunderstanding.
Energetics and stability
Stability is often used to try to explain why reactions occur. Students will be familiar with thinking about stability from their physics studies. Ask them to explain why a ball rolls down a hill, and they will likely say that it is more stable at the bottom of the hill.
It’s useful to discuss chemical stability, but we need to be aware that the concept of stability is built on the ideas of energetics, equilibrium, entropy and kinetics. While we touch on most of these points in 14–16 curriculums, there is not enough time or capacity to delve more deeply.
When discussing chemical reactions, I explain that particles are rearranging and the system is moving in an energetic landscape, generally to a position of greater energetic stability. I often draw on the analogy of a ball rolling down the hill, with systems rolling down energetic hills into stable valleys.
To appreciate this energetic landscape, we need to investigate the energy of chemical systems. Interestingly, these ideas do appear in some 14–16 physics specifications, but are not often referred to in 14–16 or 16–19 chemistry specifications.
A chemical system is made up of many particles, and these will have associated kinetic and potential energies. Combined, we call this the internal energy of the system. The kinetic energies are due to the movement of the particles (translations, rotations and vibrations) and potential energies (vibrations and electron movements). When a reaction occurs, there will be a change in the internal energy of the system, not least as the particles will be in different positions relative to each other.
A further complexity arises if there is a change in the amount of gas in the system. If more gas is produced than used up in the reaction, then the system is pushing the atmosphere out of the way. This work done on the atmosphere requires energy, and this is proportional to the change in volume.
Teaching tips
- Be upfront with students about the tricky and abstract nature of energy.
- Draw links between the sciences and use analogies for what students have learnt in physics.
- Talk about energetic stability of chemical systems in terms of how charged particles are arranged in relation to each other.
- Get your students to question the models presented in their textbooks to deepen understanding.
Energy and enthalpy
We define enthalpy as the energy transferred to or from surroundings when a reaction occurs. Enthalpy considers all the changes in kinetic energy, potential energy and gases. We also assume that the reaction occurs at a fixed temperature and pressure, allowing us to compare enthalpy changes between different reactions.
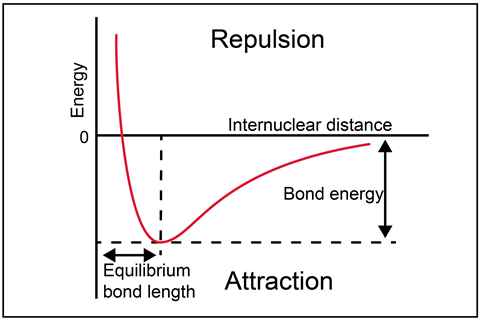
The potential energy of a chemical system is related to the charges and positions of the particles involved. Students will generally be confident to say that oppositely-charged particles are more stable closer together, and same-charged particles are more stable further away. This stability derives from the electrostatic forces between the particles, either attractive or repulsive.
Consider two hydrogen atoms (2H), far apart and then close together, bonded into a hydrogen molecule (H2). Which arrangement is more stable? Flip the question around – what do I have to do to get from H2 back to 2H? I need to put energy into the system to break the covalent bond between the atoms. As all covalent bonds have different strengths, it is convenient to set a zero point when the atoms are completely isolated – this is zero potential energy. As the atoms get closer and bond into the molecule, the system becomes more stable (energy is transferred out of the system), so we fall down the potential valley, neatly depicted in the graph.
- Discover how to build a strong foundation when introducing 11–14 learners to chemical energetics. You can also supplement 11–14 lessons on this topic with practicals examining exothermic and endothermic reactions or a quiz to develop learners’ ability to explain practical observations.
- Find out how to ensure post-16 learners have a good understanding of the concept of energy and change to build on with this guide.
- Avoid common misconceptions about the concept of chemical energetics by clarifying topic terminology.
As absolute enthalpies would be hard to determine, we determine changes in enthalpy as reactions occur. As we are most interested in what is happening to our chemical system, we define a transfer of energy from the system to the surroundings as a negative enthalpy change, and vice versa.
At 14–16, we tend to use bond enthalpies to determine enthalpy changes of a reaction. This works well for gaseous reactions involving simple covalent substances. At post-16, we cover many other types of enthalpy change, including atomisation, ionisation, electron affinity and solvation. This allows us to look at enthalpy changes involving metallic and ionic substances, and reactions in solutions.
Clearing up misconceptions
When sodium reacts with chlorine, sodium chloride is formed. Sodium is oxidised (loses electrons), chlorine is reduced (gains electrons), and sodium ions are attracted to chloride ions. Statements like the following are commonly heard: ‘the sodium is more stable when it loses an electron to get a full outer shell’. This misconception tends to be born from the ‘full outer shell’ rule of thumb which helps students to work out ionic charges and draw dot and cross diagrams.
It’s a tricky misconception to shift. Imagine an isolated sodium atom. Now remove the outer shell electron so you have a sodium ion and a free electron. This change required an energy transfer into the system – an endothermic reaction. Left alone, the electron would soon rejoin the sodium ion as there is an electrostatic attraction between them – an exothermic reaction. The sodium atom is more energetically stable than a sodium ion (full outer shell and all) and a free electron.
What ultimately drives sodium to lose the outer shell electron are the enthalpy changes of the whole reaction, including the electron being added to chlorine and the ionic lattice forming. The problem is, we use a very simplified system of an isolated sodium atom and chlorine atom. While a necessary simplification, it is probably oversimplified and embedding problematic misconceptions.
Resources for your classroom
- Use these practicals to develop student understanding of energetics and learners’ research skills.
- Use this set of activities to identify common misconceptions when teaching chemical stability.
- Look at an Institute of Physics article on the language of energy stores often used in 11–14 physics.
David Paterson













No comments yet