Share this infographic with your students, download the poster for your classroom and get students using their knowledge with the accompanying activity
Download this
This infographic is designed to be displayed as a poster in the classroom (though it could also be displayed on a projector or printed out as a handout). Use the accompanying fact sheet and differentiated flash card activity to explore the different properties and uses of four allotropes of carbon – diamond, graphite, graphene and buckminsterfullerene. Learners extract information from the infographic to complete the cards.
- Poster as pdf (A4 single pages or A3 one page)
- Fact sheet as MS Word and pdf
- Flash cards for age range 14–16 as MS Word or pdf
- Flash card answers as MS Word or pdf
View and download more infographics
Some elements are able to exist in different structural forms, known as allotropes. Carbon does this very well because of its ability to form bonds with other neighbouring carbon atoms – something called catenation.
The way in which carbon atoms are connected to each other makes a big difference to the physical, chemical and electronic properties of the material. Some carbon allotropes have been known for a long time; others have been discovered more recently.
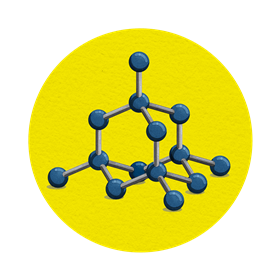
Diamond
Structure
Diamond has a tetrahedral structure. Each carbon atom is connected to four other carbon atoms by a covalent bond to form a giant crystal lattice.
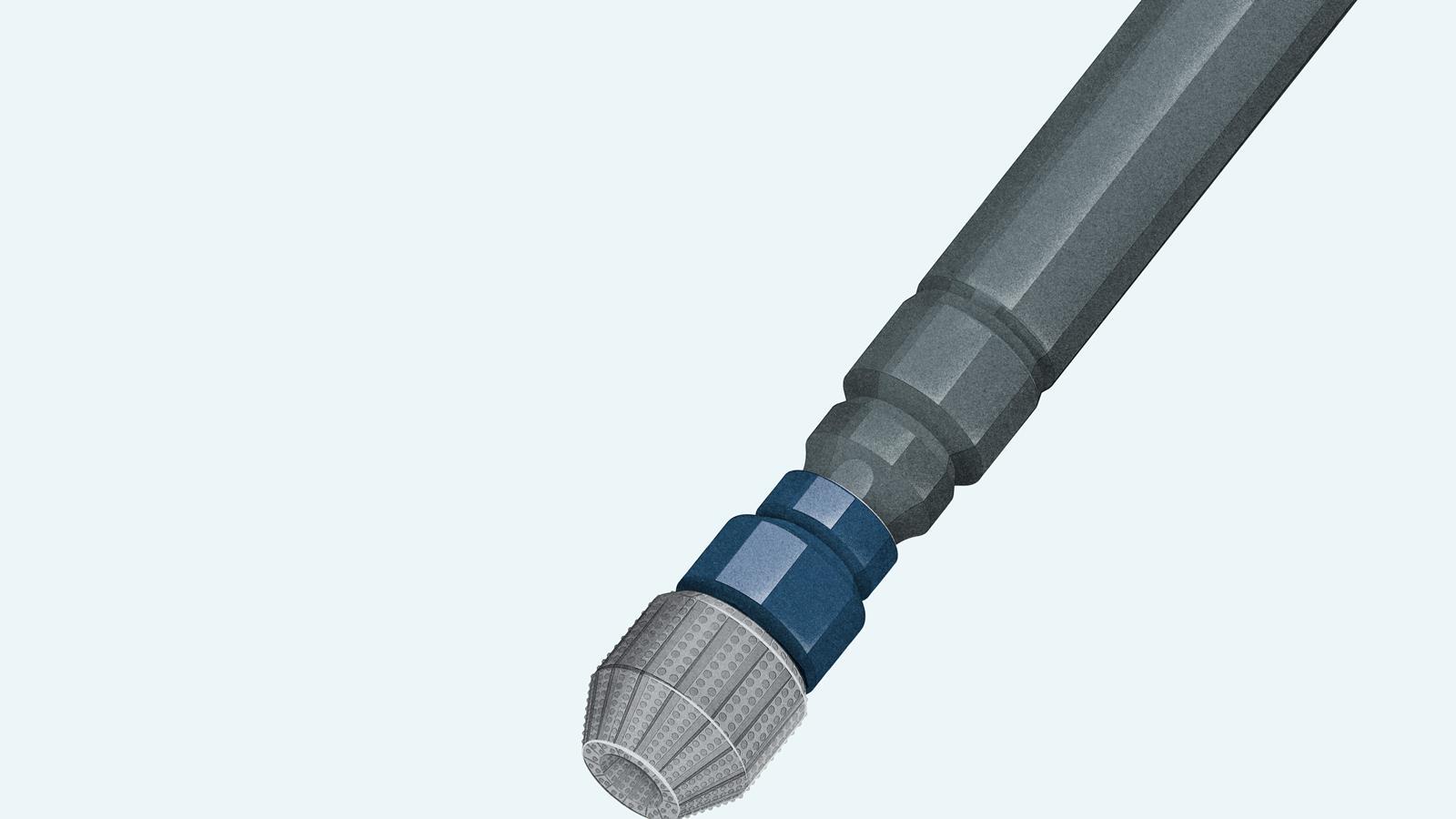
Used for …
- drill bits in oil exploration and for slicing through concrete
- jewellery: naturally-made diamonds are of higher purity and very expensive!
Because …
- of its tetrahedral structure, diamond is one of the hardest known materials
- it has a high refractive index, light is reflected internally, so it sparkles
- all diamond’s electrons are used to create the bonding lattice, leaving none spare, it’s a poor conductor of electricity
4027°C – That’s how much you have to heat diamond to break all its bonds and liquefy it into molten carbon. It’s so hard to change into a gas because of its tetrahedral structure.
3600°C – Heat graphite to this temperature to sublime it. That’s how much energy it takes to break its covalent bonds. Perfect for nuclear reactor cores.
Graphite
Structure
Each carbon atom is covalently bonded to just three others, leaving one electron spare. This results in atoms arranged in flat layers of hexagons, between which is a soup of free, delocalised electrons that’s made up from the spare electrons.
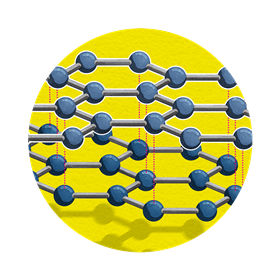
Used for …
- pencil leads
- nuclear reactor cores, to stop or slow the nuclear reaction
Because …
- its layer-like structure makes it soft and flaky, as a pencil it leaves marks on your paper
- so much energy is needed break the covalent bonds, graphite is tough enough to be used in a nuclear reactor
- of its soup of spare electrons, graphite is a very good conductor of electricity
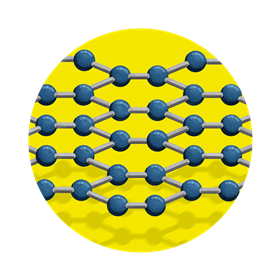
Graphene
Graphene was a theoretical concept before it was isolated and studied in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester, who were awarded the Nobel prize in physics in 2010 for their discovery. It’s the thinnest, lightest, strongest, most stretchy material we’ve ever created.
Structure
Think of graphene as a single layer extracted from graphite. In its hexagonal lattice, each carbon is bonded with three others, leaving a spare electron.
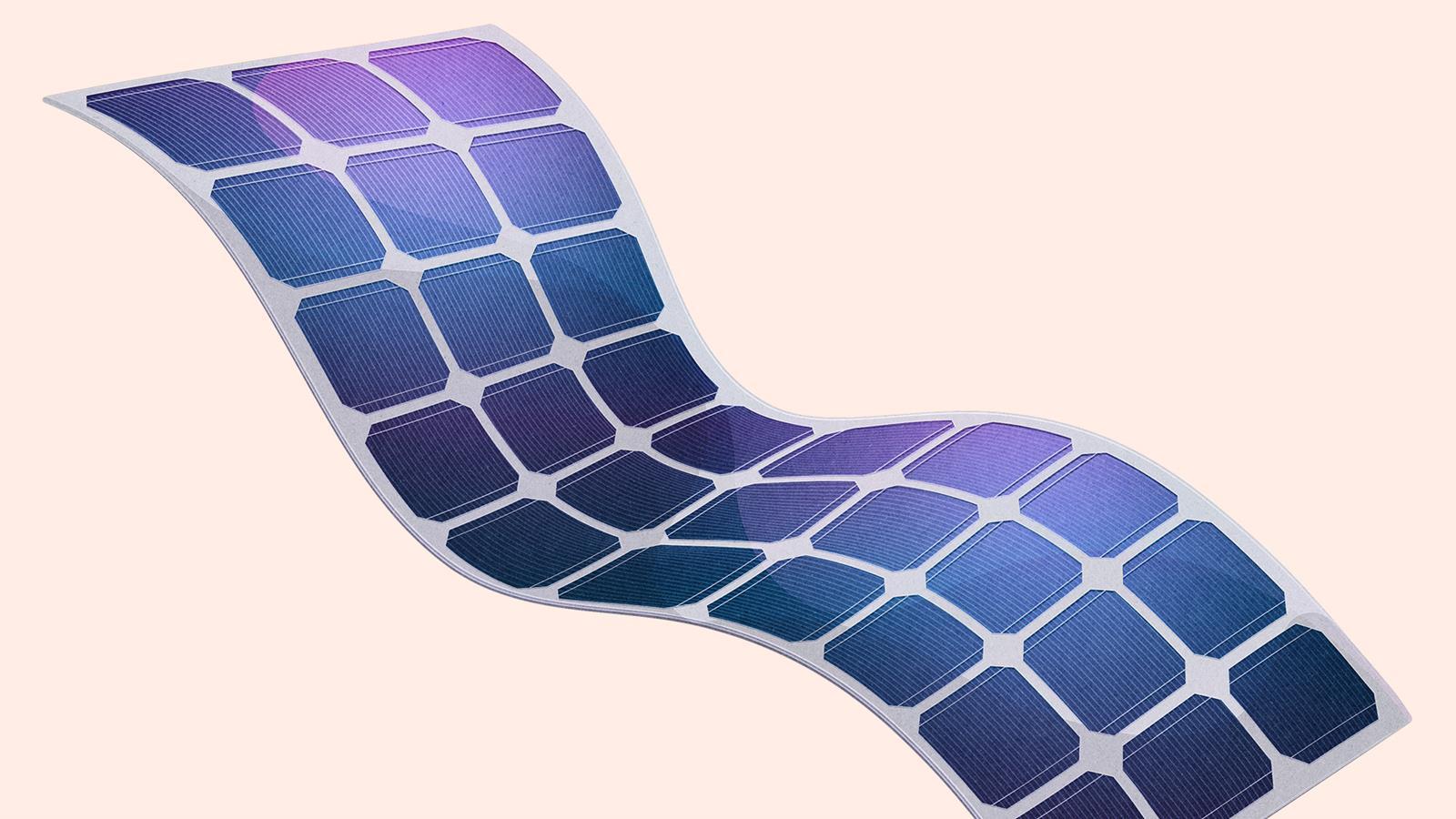
Used for …
- solar cells that are both transparent and flexible
- smart windows that can control heat and light transmittance
- electronic displays
Because …
- of its spare electrons graphene is an excellent conductor of electricity and heat
1 g – that's how much a single sheet of graphene the size of a football pitch would weigh!
In 1985, a team headed by Professor Sir Harry Kroto from the University of Sussex discovered and named C60 or buckminsterfullerene.
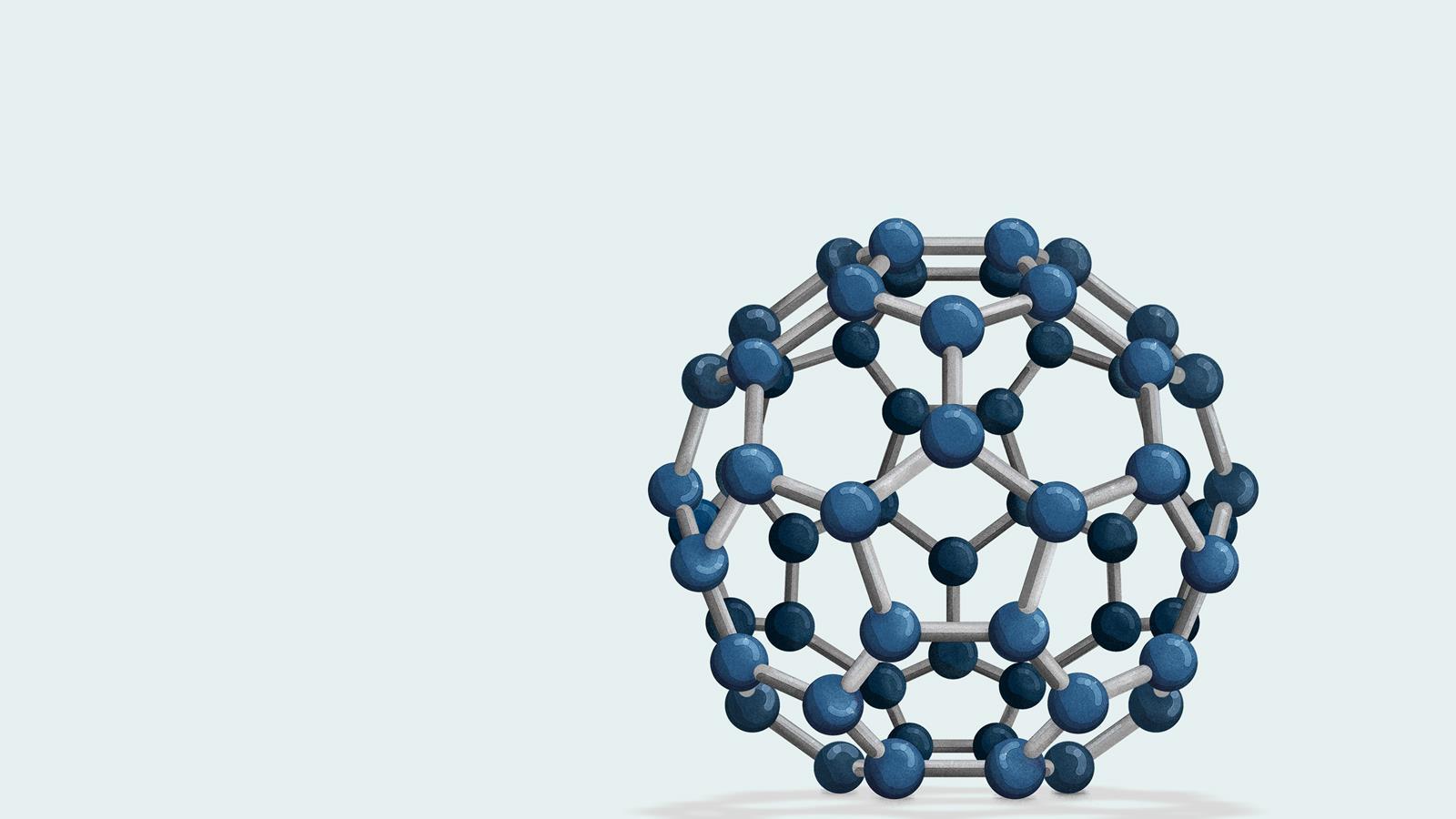
Fullerenes
Until 1985 it was thought there were only two allotropes of carbon: diamond and graphite. But scientists thought they had detected the presence of another form of carbon in space. That mysterious new allotrope is C60, or buckminsterfullerene. Other fullerenes exist too, like C70, as well as ellipsoids and tubes.
Structure
Buckminsterfullerene’s spherical structure comprises 60 carbon atoms arranged as 20 hexagons and 12 pentagons. The same shape as a football – which is why C60 is also sometimes called a ‘buckyball’.
Used for …
- (potentially) drug delivery – many researchers are currently working on this
Because …
- buckminsterfullerene’s intermolecular forces are weak, its melting point is low
- fullerenes have a sea of free electrons inside, they can conduct electricity
All illustrations © Dan Bright
Downloads
Allotropes of carbon infographic poster A4
Handout | PDF, Size 1.25 mbAllotropes of carbon infographic poster A3
Handout | PDF, Size 1.18 mbFact sheet allotropes of carbon
Editable handout | Word, Size 78.38 kbFact sheet allotropes of carbon
Editable handout | PDF, Size 32.22 kbFlashcards allotropes of carbon student
Editable handout | Word, Size 0.22 mbFlashcards allotropes of carbon student
Handout | PDF, Size 0.22 mbFlashcards allotropes of carbon answers
Editable handout | Word, Size 0.22 mbFlashcards allotropes of carbon - answers
Handout | PDF, Size 0.22 mb


































3 readers' comments