Use these ideas and activities to help embed the understanding of reduction and oxidation with your students
It is quite likely that the legs of the tables and chairs in your classroom are made of iron. If your students were to ask you where this iron has come from, as a chemist, you have a much more interesting answer to give them than ‘from a shop’.
The story of where iron comes from and how we ended up using it in our furniture shows the day-to-day impact of the chemistry of metals. Iron ore has been mined, reduced and shaped to produce our chair and table legs. The copper in our phones and lead in car batteries has undergone similar processes.

On a walk through Lickey Hills Country Park near Birmingham, I admire the view: the stark contrast of hills, trees and fields close by and the concrete, glass and metal of Birmingham skyline behind. Although not obvious to all, metals are in plentiful supply close by, albeit in their oxidised form as positive metal ions. Magnesium in the chlorophyll of the leaves, calcium and many other metal ions in the earth beneath my feet to name but a few locations. By reducing metal ions in compounds, we can extract metals for our use and literally build the world around us.
What students need to know
• An ore is a mineral that contains enough metal to make it worth extracting the metal. Carbon can be used to extract metals from some metal oxides.
• A metal can either be reduced or oxidised in a reaction.
• Reduction occurs when a metal in a compound loses oxygen, to form the elemental metal.
• Oxidation occurs when a metal (or carbon) gains oxygen, to form an oxide compound.
Ideas for the classroom
Many metals can be reduced and extracted in a school laboratory. The easiest are iron, copper and lead. Over several lessons students can be taught how a metal is extracted and reduced from its ore. As you introduce each new example, increase the complexity of the chemistry. This process will continue during GCSE and A-level studies. Your teaching of reduction and oxidation, or redox, at 11-14 creates a conceptual bedrock upon which the rest is built.
Begin with students identifying metals that are oxidised (those in compounds with oxygen) or reduced (as elements). Show examples including metal ores and compounds and contrast these with metals themselves. Ask students to identify the oxidised and reduced forms. Discuss the different properties linking back to metals and non-metals, compounds and elements and ions and atoms.
Begin with students identifying metals that are oxidised (those in compounds with oxygen) or reduced (as elements). Show examples including metal ores and compounds and contrast these with metals themselves. Ask students to identify the oxidised and reduced forms. Discuss the different properties linking back to metals and non-metals, compounds and elements (rsc.li/354CsQJ) and ions and atoms (rsc.li/2Pt75sM).
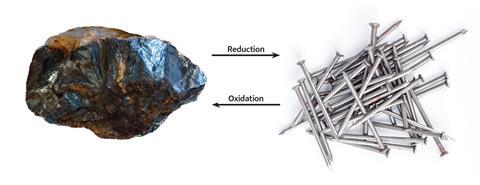
Build up your students’ understanding by progressively increasing the detail of the concepts. Use the following definitions in the sequence whilst interleaving prior knowledge:
| Reducing a metal | Oxidising a metal | |
|---|---|---|
|
1st |
Removal of oxygen |
Addition of oxygen |
|
2nd |
Forming an element |
Forming a compound |
|
3rd |
Forming an atom |
Forming an ion |
Challenge students to describe the reactions using as many keywords as possible to build their fluency. Ask them to include the words reduced, reducing, reduction, oxidised, oxidising and oxidation as many times as possible while still making sense. For example, for the equation: iron oxide + carbon → iron + carbon dioxide, they could say: ‘the oxidised iron in the iron oxide is reduced by the carbon which is oxidised to form the oxidised form of carbon which is carbon dioxide. The reduced iron is left by itself’.
This sequencing can be introduced through a series of questions; see some in the worksheets below.
Download this
Worksheets on metal extraction, teachers notes and answers from the Education in Chemistry website: rsc.li
Practical chemistry
Students can perform reduction experiments themselves such as reducing iron oxide to iron. A favourite class practical lesson is extraction of iron on a match head. Begin by showing a sample of the iron ore haematite. Discuss sources of iron ore and mines such as the former Florence iron mine in Egremont, Cumbria which closed in 2008. I tell my students how ‘I spent hours with a hammer crushing the haematite until it is a fine powder for them to use’. Then show them the powdered iron oxide.
Students can perform reduction experiments themselves such as reducing iron oxide to iron. A favourite class practical lesson is extraction of iron on a match head (rsc.li/2P4VEIT). Begin by showing a sample of the iron ore haematite. Discuss sources of iron ore and mines such as the former Florence iron mine in Egremont, Cumbria which closed in 2008. I tell my students how ‘I spent hours with a hammer crushing the haematite until it is a fine powder for them to use’. Then show them the powdered iron oxide.
Dip a match head successively in water, then sodium carbonate and finally the iron oxide before burning the mixture. Crush the burnt mixture with a spatula in a weighing boat and use a magnet underneath to show iron has been produced. The wood of the match acts as the source of carbon, reducing the iron oxide to iron. Students should be able to write the word equation and identify reduction and oxidation as described above.
Lead can be reduced from its ore galena (lead sulfide), which used to be mined in Shropshire. Copper can be reduced from malachite (copper carbonate hydroxide), which was mined in the ancient copper mines at Great Orme’s Head, Llandundo. Both ores require roasting – heating in air – before they are ready to be reduced. This step can be modelled in the classroom by the thermal decomposition of copper carbonate into copper oxide before proceeding to reduce with carbon.
Lead can be reduced from its ore galena (lead sulfide), which used to be mined in Shropshire. Copper can be reduced from malachite (copper carbonate hydroxide), which was mined in the ancient copper mines at Great Orme’s Head, Llandundo. Both ores require roasting – heating in air – before they are ready to be reduced. This step can be modelled in the classroom by the thermal decomposition of copper carbonate into copper oxide (rsc.li/2rsYsX5) before proceeding to reduce with carbon (rsc.li/2sY6bNb).
Formative assessment
Students should now be able to transfer their knowledge of the reduction of iron oxide to the examples of copper oxide and lead oxide. Ask them to explain how elemental copper and lead are formed from the ores. Use individual whiteboards to give students a low-stakes method for working out their thinking, and to receive quick individual verbal feedback. The worksheets can be used to assess students’ proficiency. Model your thinking as you go through one set of answers, then ask students to self- or peer-assess the other answers.

Progression 14-16
The concept of reduction and oxidation can be expanded beyond the reduction of metals. The next steps will include describing reactions in terms of electron transfer.
For instance, display a rusting iron chain or the copper statue of Queen Victoria in Birmingham. Ask students: what has happened to both the iron in the chain and to the copper in the statue?
Discuss what happens to the non-metal in a compound when the metal is reduced. Does it remain as part of a compound and is therefore neither reduced nor oxidised? Or is it removed from the compound and therefore oxidised?
Common misconceptions
A common misconception is that oxidation is limited to a reaction with oxygen and reduction limited to the removal of oxygen. Take care not to offer restrictive definitions of reduction or oxidation – highlight that many key terms in the sciences can have multiple, often overlapping, meanings.
Be clear with what is meant by ‘with oxygen’. Highlight the difference between a metal oxide compound, such as iron oxide, and a mixture of the elements, such as iron and oxygen.
Non-metals can react in the opposite way to metals when extracted by redox, which can cause confusion – for example chlorine is oxidised when extracted from sodium chloride solution.
Take-home points
- Metals can be extracted from ores by reduction – the removal of oxygen or forming a metal element from a compound.
- Oxidation and reduction have multiple meanings, not just to do with the addition and removal of oxygen.
- Encourage students to use key terms as often as possible. Constant modelling of keywords will help build students’ confidence and fluency.
- Students’ understanding of redox will be developed into their understanding of electron transfer and reactions including displacement and electrolysis.









No comments yet