Mechanisms before reactions
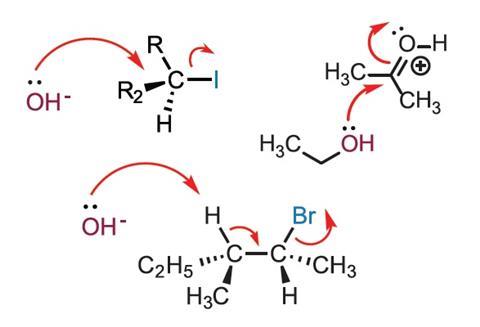
To the uninitiated, organic chemistry can appear to be like a vast repository of loosely connected facts requiring extensive rote-memorisation. Such thinking is encouraged by traditional modes of teaching, which typically introduce the functional group concept and reaction sequences before students understand the underpinning principles of structure and reactivity. In their recent article, Alison Flynn and William Ogilvie describe a new approach, ‘Mechanisms before Reactions’, built around a mechanistic approach that focuses on understanding of the concept of electron flow and its role in organic reactivity.
The authors note that most textbooks do place an emphasis on reaction mechanisms and the electron-pushing formalism (EPF), but that they remain organised around ‘functional groups’. This can lead to rote-memorisation since complex topics are often presented before simple concepts have been grasped. This undoubtedly contributes to the view held by many students that organic chemistry is random and difficult. Conversely, a student grasping the EPF may develop into an effective organic chemist – they can apply their understanding of mechanism to predict reactions and devise synthetic strategies.
The article describes the redesign of two organic chemistry modules taught to more than 1000 students at the University of Ottawa, Canada. In the new approach, the concept of mechanism is taught before students learn a single reaction, reactions are organised by their governing mechanism and initial teaching aims to develop the skills needed to explain and predict mechanisms. The fundamentals covered at the beginning included line structures, physical properties, conformational analysis and stereochemistry. Electron-pushing is introduced with the aim of ensuring students are able to interpret and use curly arrows to predict and draw products of reactions. In teaching, the mechanisms are colour-coded to help students visualise the movement of electron pairs. The first reactions covered are simple proton transfers, with subsequent mechanisms being taught in order of difficulty.
Although a formal evaluation is not presented, it is reported the approach has been well received by students and staff alike. The authors note that students performed well on examination questions featuring reactions they had not seen previously, and they are currently carrying out an investigation into the impact on learning. With the return of terminal assessment to UK A-levels, teachers will be freed from the constraints of modularisation and such approaches may be of value in the classroom.
References
A B Flynn and W W Ogilvie, J. Chem. Ed., 2015, 92, 803 (DOI: 10.1021/ed500284d)









No comments yet