In this post, I give some detail of the Context Based Learning symposium from the recent International Conference on Chemical Education (ICCE).
![Shutterstock 167119931 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/480xAny/7/0/1/113701_shutterstock_167119931_300tb1.jpg)
Context based learning (CBL) is a popular method of teaching and it was no surprise to see that a symposium with three sessions was dedicated to this approach at the recent International Conference on Chemical Education meeting (ICCE). This symposium was organised by Peter Mahaffy and Ilka Parchmann, both of whom have done tremendous work in the area of context based learning.
Context based learning aims to teach chemical concepts by starting with observations from real world contexts and relating them to the molecular and symbolic representations with which we typically describe chemical phenomena. The rationale for this approach is that it is more interesting and thus motivating for students, and it can use the students’ prior knowledge so they may build on their scientific literacy. A good example using climate science was mentioned in a talk by Lallie McKenzie, a collaborator of Mahaffy’s, where introductory level students are shown earth temperature and CO2 levels over a long period of time as an opening discussion point. This is used to initiate questions on how the earth’s temperature and levels of CO2 can be measured, leading to discussion of isotopes and isotope ratios in ice cores (more on this wonderful project in September).
While CBL has acknowledged advantages, the difficulty is that students have to simultaneously consider the chemical concepts as well as the contextual situation, and thus it may prove more challenging learners and require them to operate at a higher level of thinking. Parchmann’s opening keynote to the context based learning symposium described work on how best to design CBL exercises for school children where the points of difficulty could be identified, and appropriate scaffolding and hints detailed. The work has recently been described more fully by Broman and Parchmann in Chemistry Education Research and Practice (free to access).
Some interesting things came out of the presentation for me. The meaning of context was elaborated on. Rather than just an example added on to a traditional representational chemistry approach, Parchmann outlined a systematic design of a context based scenario. This involved considering the content area (e.g. organic chemistry in relation to chemical bonding), the topic (e.g. medical drugs, energy drinks, fats) within this content area, and then framing these topics within a context: a personal context, a societal context, and a professional context (see Figure from CERP, reproduced with permission). These were provided to the students in everyday language.
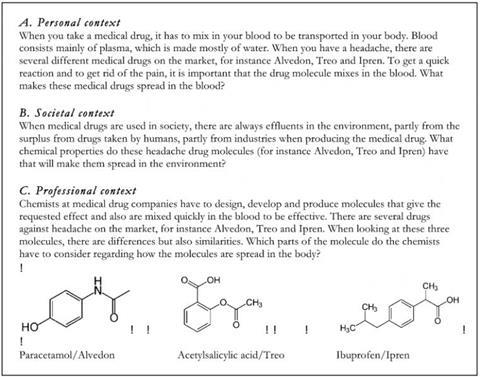
The students found the contexts relevant and interesting, especially so with the personal contexts. The tasks were unfamiliar to them; they reported that they differed a lot from the typical school chemistry tasks, where they “knew the answer the teacher was looking for.” Because of the open and broad nature of the problems—in terms of goal clarity and structuring the response—guidance is needed as the students work through the problem. An interesting observation in this regard was that some students were shown the molecular structures along with the question. These students typically looked immediately for a familiar chemistry concept (e.g. the presence of an –OH group) and used that as a basis to provide an answer (e.g. to discuss solubility)—they were addressing it using a problem-solving approach and looking for the single factual answer. Those students who were not provided with the structure until later on in the task tended to discuss the concepts more broadly. In addition, students tended to use words within the context provided to help structure their own answer.
In terms of the process of teaching, four phases were identified: (i) the phase of contact, where the relevance of the problem is outlined and links to pre-knowledge made; (ii) the phase of curiosity and planning, where students develop questions so that they can approach the problem; (iii) the phase of development, where the students complete activities to explore and develop their ideas, and finally (iv) the phase of deepening, where the relevance of the chemistry can be incorporated. It is in the latter phase where the teacher has an important role.
I liked the presentation as it provided a useful structure on how to integrate context based learning into teaching. With the increasing prevalence of context-based materials out there, it is useful to consider such a structure so that they may be meaningfully incorporated into our teaching.
Notes:
- K. Broman and I. Parchmann, Students’ application of chemical concepts when solving chemistry problems in different contexts, Chemistry Education Research and Practice, 2014, doi: 10.1039/c4rp00051j
- The RSC Learn Chemistry site has several context- and problem-based learning resources, available at this link: http://www.rsc.org/learn-chemistry/resource/listing?searchtext="CandPBL"









No comments yet