Use these approaches to ensure your students develop good mental models of the atom and avoid common misunderstandings
Imagine firing a gun at a sheet of paper, only for the bullet to ricochet straight back towards you. This startling (and potentially fatal) scenario reflects just one of the counterintuitive ideas students encounter when learning about atomic structure. It’s a topic that challenges students to adopt new ways of thinking as they explore the fundamental building blocks of matter.
Atomic structure is built around models, which can sometimes confuse students
Atomic structure is a cornerstone of the 14–16 curriculum that underpins the entire discipline of chemistry. Mastering this topic equips students to understand chemical bonding, reactivity and the organisation of the periodic table — core principles of the subject.
What students need to know
By the end of this topic, students should have a solid grasp of the structure of the atom, its subatomic particles and how atomic structure relates to the periodic table. At 11–14, students typically learn that matter is made of atomsand that particles behave differently in solids, liquids and gases. However, the internal structure of the atom is generally a new concept for ages 14–16, when we introduce learners to several key ideas:
- Atoms are composed of protons, neutrons and electrons. Protons are positively charged, neutrons are neutral and electrons are negatively charged.
- Protons and neutrons are located in the nucleus, whereas electrons occupy shells around it.
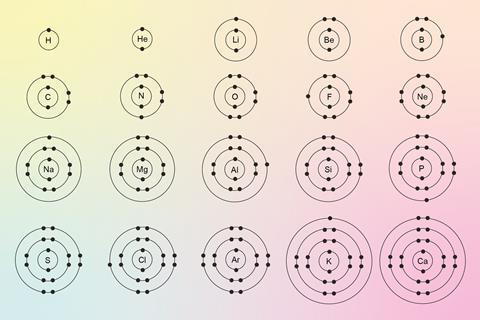
- The first shell can hold up to two electrons and the second and third can hold up to eight each. For example, the configuration is 2,8,8,2 for calcium.
- The atomic number represents the number of protons, but the mass number is the sum of protons and neutrons.
- Atoms are neutral overall because they contain equal numbers of protons and electrons. Ions form when atoms gain or lose electrons.
- Atomic structure is intrinsically linked to the periodic table. For example, elements in group 1 all have one electron in their outer shell.
- Isotopes are atoms of the same element with different numbers of neutrons.
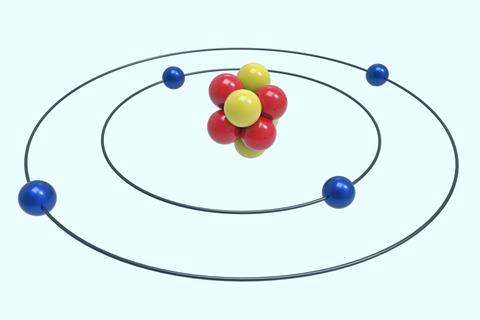
Teach these ideas incrementally and provide plenty of opportunities for review and retrieval of key concepts.
Atomic structure is built around models, which can sometimes confuse students if they are presented as definitive or literal representations, rather than tools to explain observations and predict behaviours. It’s vital to help students appreciate that scientific understanding evolves through learning a series of models, each one more refined than the last and to improve our grasp of the natural world. Historical models, such as Dalton’s indivisible atom, Thomson’s plum pudding model, Rutherford’s nuclear model and Bohr’s orbital theory (rsc.li/4hmpuTG), provide an excellent framework to illustrate how scientific ideas evolve through testing and refinement.
Atomic structure is built around models, which can sometimes confuse students if they are presented as definitive or literal representations, rather than tools to explain observations and predict behaviours. It’s vital to help students appreciate that scientific understanding evolves through learning a series of models, each one more refined than the last and to improve our grasp of the natural world. Historical models, such as Dalton’s indivisible atom, Thomson’s plum pudding model, Rutherford’s nuclear model and Bohr’s orbital theory, provide an excellent framework to illustrate how scientific ideas evolve through testing and refinement.
Encourage students to see the current atomic model as a simplified representation. Although it is good enough to explain bonding and reactivity at this level, they will unravel the idea more if they go on to study chemistry at a higher level.
What you need to know
At post-16, students will learn that the shells or energy levels they are visualising as circles are actually comprised of subshells (rsc.li/3Erfpq5). They will also learn that electrons don’t follow set paths around an atom – we can never be certain of both the position and the momentum of an electron. What’s more, we’re not even sure what electrons are despite us explaining them as subatomic particles. We can describe them as both particles and waves.
At post-16, students will learn that the shells or energy levels they are visualising as circles are actually comprised of subshells. They will also learn that electrons don’t follow set paths around an atom – we can never be certain of both the position and the momentum of an electron. What’s more, we’re not even sure what electrons are despite us explaining them as subatomic particles. We can describe them as both particles and waves.
At 14–16, introduce shells as regions in which electrons are likely to be rather than thinking of them as following fixed paths. Be clear that all these ideas about atoms and electrons are models. These approaches lay the foundation for more advanced ideas, such as quantum mechanics and electron configurations and can help pre-empt misconceptions.
Common misconceptions
Learners may think of electron shells as rigid, physical structures surrounding the nucleus, or visualise atoms as miniature solar systems with electrons orbiting the nucleus like planets. Some believe that atoms are solid spheres, rather than mostly empty space. You can address these misconceptions by:
- using diagnostic questions to identify and address errors in understanding. The RADAAR framework is a useful tool for planning such activities.
- exploring the similarities and differences between the planetary models of the atom and the solar system using this analogy resource.
- demonstrating the scale of atoms to show students just how small the nucleus is relative to the atom it’s in. For example, compare the nucleus to a marble inside a football stadium for a sense of its size relative to that of an atom. Rutherford’s famous analogy for his gold foil experiment is also effective: ‘It was almost as incredible as if you fired a 15 inch shell at a piece of tissue paper, and it came back and hit you.’
Suggestions for teaching
Students often confuse atomic mass and atomic number, or struggle to identify which particles these represent. Regular retrieval quizzes, along with activities that reinforce links between atomic structure and the periodic table, can help embed these ideas. Understanding and recalling these links needs to be automatic, because students will need to apply these ideas for more complex topics later.
It’s also important to explicitly teach why atoms are neutral. You may need to remind learners that protons and electrons have opposite charges, and that these charges cancel each other out (if there is an equal number of both) or leave a charged particle (if this isn’t the case). Bar models can be a helpful visual tool to show the balance of positive and negative charges, as well as the imbalance in charges that arises when electrons are gained or lost to form ions.
It’s important that, by the end of this topic, students are confident with drawing electron configurations and linking this to the structure of the periodic table. Fluency in reading the periodic table and applying this knowledge to elements is essential for understanding reactivity and bonding.
By linking atomic structure to the broader context of chemistry, reinforcing key ideas through repetition and addressing potential pitfalls head-on, you can ensure students build a solid understanding of this fundamental topic.
Resources for your classroom
See all of our supporting resources for atomic structure at a glance in our atomic model resources package for learners aged 14–16.
- Make your own Top Trump cards for exploring the periodic table.
- Download and display the step-by-step guide to drawing electron configurations.
- Scaffold writing for learners with this structure of the atom resource.
- Build an atom with this interactive resource and read more about using similations to help reinforce the links between subatomic particles, atomic structure and the periodic table.
- Show students how a career in research can allow them to work with atomic and subatomic particles.













No comments yet