In this experiment, a known mass of hydrated copper(II) sulfate is heated to remove the water of crystallisation
This is a class experiment suitable for students who already have a reasonable understanding of the mole concept.
The mass of water is found by weighing before and after heating. This information is used to find x in the formula CuSO4.xH2O, using mole calculations
The degree to which the mole calculations need to be structured will depend on the ability and mathematical competence of the class. The outline structure given in the Procedure above is intended for students with reasonable mathematical competence and experience of mole calculations.
Given adequate access to top-pan balances, and skill in their use, students should be able to complete the experimental work in 30–40 minutes.
Equipment
Apparatus
- Eye protection
- Crucible (note 1)
- Crucible tongs (note 2)
- Tripod
- Pipe clay triangle
- Bunsen burner
- Heat resistant mat
- Top-pan balance (± 0.01 g)
Apparatus notes
- Crucibles may be of porcelain, stainless steel or nickel, of capacity about 15 cm3, and should sit safely in the pipe clay triangles provided.
- Crucible tongs should have a bow in the jaws of the right size to pick up the hot crucibles safely.
Chemicals
- Hydrated copper(II) sulfate (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), 2–3 g
Health, safety and technical notes
- Read our standard health and safety guidance
- Hydrated copper(II) sulfate, CuSO4.5H2 O(s), (HARMFUL, DANGEROUS FOR ENVIRONMENT) – see CLEAPSS Hazcard HC027c. The copper(II) sulfate should be provided as fine crystals. If large crystals are used, these should be ground down before use by students.
Procedure
- Weigh the empty crucible, and then weigh into it between 2 g and 3 g of hydrated copper(II) sulfate. Record all weighings accurate to the nearest 0.01 g.
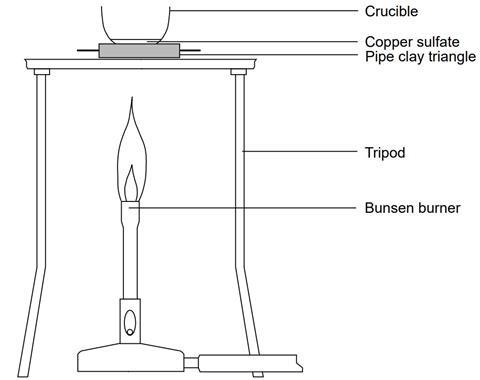
- Support the crucible securely in the pipe-clay triangle on the tripod over the Bunsen burner.
- Heat the crucible and contents, gently at first, over a medium Bunsen flame, so that the water of crystallisation is driven off steadily. The blue colour of the hydrated compound should gradually fade to the greyish-white of anhydrous copper(II) sulfate. Avoid over-heating, which may cause further decomposition, and stop heating immediately if the colour starts to blacken. If over-heated, toxic or corrosive fumes may be evolved. A total heating time of about 10 minutes should be enough.
- Allow the crucible and contents to cool. The tongs may be used to move the hot crucible from the hot pipe-clay triangle onto the heat resistant mat where it should cool more rapidly.
- Re-weigh the crucible and contents once cold.
- Calculation:
- Calculate the molar masses of H2O and CuSO4 (Relative atomic masses: H=1, O=16, S=32, Cu=64)
- Calculate the mass of water driven off, and the mass of anhydrous copper(II) sulfate formed in your experiment
- Calculate the number of moles of anhydrous copper(II) sulfate formed
- Calculate the number of moles of water driven off
- Calculate how many moles of water would have been driven off if 1 mole of anhydrous copper(II) sulfate had been formed
- Write down the formula for hydrated copper(II) sulfate.
Teaching notes
Remind students to zero (tare) the balance before each weighing.
Students will probably also have to be reminded about the need to allow the crucible and contents to cool thoroughly before weighing.
Metal crucibles (stainless steel or nickel) are much less vulnerable than porcelain crucibles.
More resources
Add context and inspire your learners with our short career videos showing how chemistry is making a difference.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















4 readers' comments