- Home
- I am a …
- Resources
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- Literacy in science teaching
- More …
- Climate change and sustainability
- Alchemy
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Collections
- Education in Chemistry
- Teach Chemistry
- Events
- Teacher PD
- Enrichment
- Our work
- More navigation items
Experiments
Try these experiments in your class to develop students’ practical science skills and explore key chemical reactions and processes
- Class experiment
Heating chocolate and egg
Introduce learners to physical and chemical changes and the safe use of Bunsen burners with this simple practical experiment
- Class experiment
Iron and sulfur reaction
Illustrate elements, mixtures and compounds with this demonstration and class practical exploring the exothermic reaction of iron and sulfur
- Class experiment
The change in mass when magnesium burns
A class practical to find the formula of magnesium oxide using the change in mass when magnesium burns
- Class experiment
A microscale acid–base titration
Use microscale titration to complete an acid–base neutralisation with sodium hydroxide in this class practical. Includes kit list and safety instructions.
- Class experiment
Heating copper in air
Explore the reaction of copper with oxygen to produce copper oxide by heating a copper envelope in this practical
- Class experiment
Electrolysis of brine
Use this colourful practical to introduce learners to the electrolysis of brine, or sodium chloride solution. Includes kit list and safety instructions.
- Class experiment
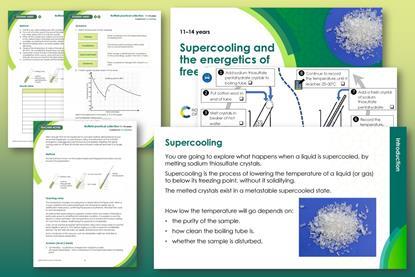
Supercooling and the energetics of freezing
Explore what happens when a liquid is supercooled and develop learners’ observation skills in this class practical
- Class experiment
Viscosity | Classic chemistry experiments | 11–14 years
Compare the viscosity of thick and thin liquids by measuring the time is takes for a bubble to move through them
- Class experiment
Particles in motion? | Classic chemistry experiments | 11–14 years
Explore the movement of gas particles by reacting calcium carbonate with hydrochloric acid and testing with limewater
- Class experiment
Experiments with particles | Classic chemistry experiments | 11–14 years
Use common classroom items to explore physical states and how materials interact in these three practical investigations
- Class experiment
Dissolved substances in tap water and seawater
Compare the solids and gases dissolved in tap water and seawater in this class practical and demonstration
- Class experiment
Rate of evaporation
Use this class practical to measure and compare the rate of evaporation of propanone under different conditions
- Class experiment
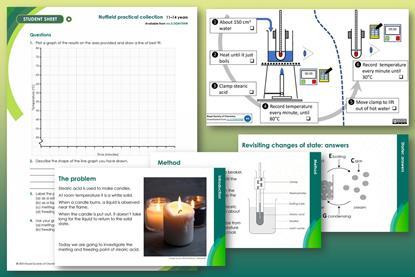
Melting and freezing stearic acid
In this class practical students take the temperature of stearic acid at regular intervals as they heat and cool it. Includes kit list and safety instructions.
- Class experiment
Acid–base back titration | 16–18 years
Write balanced equations and calculate reacting masses and moles to find the limiting reagent
- Class experiment
‘Gold’ coins on a microscale | 14–16 years
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet
- Class experiment
Acid–alkali conductometric titration worksheet | 14–16 years
Develop your learners’ understanding of ions and the changes in ionic concentrations in an acid-alkali neutralisation
- Class experiment
Practical potions microscale | 11–14 years
Observe chemical changes in this microscale experiment with a spooky twist.
- Class experiment
Antibacterial properties of the halogens | 14–18 years
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Class experiment
Air pressure, gases and the leaky bottle
Try this simple investigation to explore the effects of air pressure, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Properties of gases, air pressure and ‘sticky’ cups
Try this investigation to explore the effect of heat on gases, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Dissolving, density and sugary drinks
Try this investigation to get learners exploring the mass of sugar dissolved in their favourite drinks, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Irreversible changes and the ‘freaky hand’
Try this investigation to get learners thinking about when an irreversible reaction produces a gas. Includes detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Irreversible changes and the ‘fire extinguisher’
Try this investigation to explore what materials need to burn, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Properties of solids and ‘biscuit bashing’
Try this investigation to observe the properties of granular solids, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Fizzy irreversible changes and bath bombs
Try this investigation to explore irreversible reactions, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Air pressure and the antigravity bottle
Try this investigation exploring air pressure and gravity, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Viscosity and ‘racing’ liquids
Try this investigation to get learners talking about properties of different liquids, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Freezing and the ‘intriguing ice’ experiment
Try this investigation to explore how materials change when they freeze, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Liquids, gases and the ‘lava lamp’
Investigate liquids and gases, plus practise taking accurate measurements, with detailed teacher notes, classroom slides and a video demonstration.
- Class experiment
Equilibrium and Le Chatelier’s principle
Investigate the effects of concentration, pressure and temperature on equilibrium and explore Le Chatelier’s principle in this series of demonstrations.
- Class experiment
How to purify water
Explore the water cycle, and see how it can be used to purify your own water. A perfect experiment for the classroom, or at home, with kit list and safety instructions included.
- Class experiment
Investigating surface tension with milk
Using simple household equipment, you can explore surface tension with learners, and create amazing patterns in milk. Includes kit list and safety instructions.
- Class experiment
Kitchen roll chromatography
Coloured inks can be a mix of a few different colours, and with chromatography, learners can actually see which colours come together to make that coloured ink. This experiment includes a kit list, and safety instructions.
- Class experiment
How to make butter
Make science delicious with this experiment on how to make butter! Explore how fat molecules bind, and emulsions are created.
- Class experiment
Tricking taste buds with toothpaste
Keep tongues wagging as you explore the science of your tongue! Toothpaste can alter how our taste buds work, and learners will discover how to trick them.
- Class experiment
Making bath bombs
Learn how to make fizzing bath bombs using ingredients from your kitchen cupboards. Includes a video aimed at learners, kit list, instructions and explanation
- Class experiment
Red cabbage rainbows
In this activity, learners create rainbows using homemade red cabbage indicator paper. Includes video aimed at learners, kit list, instruction and explanation
- Class experiment
Separation techniques
An experiment into separating solids from liquids, using a range of simple household objects. Includes kit list, safety instructions and a great instructional video
- Demonstration
The equilibrium between two coloured cobalt species
In this demonstration the equilibrium between two different coloured cobalt species is disturbed. Le Chatelier’s principle is used to predict a colour change.
- Class experiment
Precipitation reactions of lead nitrate
Compare the colours of various lead compounds to identify which would be good pigments in this microscale practical. Includes kit list and safety instructions.
- Class experiment
Some reactions of sulfur dioxide
Observe the reactions of sulfur dioxide with potassium manganate (IV), iodide/iodate mixture and indicator solution. Includes kit list and safety instructions.
- Class experiment
The determination of copper in brass
Try this microscale class practical to investigate how much copper there is in brass using nitric acid. Includes kit list and safety instructions.
- Class experiment
Microscale reactions of hydrogen sulfide
Observe the reactions of hydrogen sulfide with lead nitrate, silver nitrate and potassium manganate(VII) in this microscale practical. Includes kit list and safety instructions.
- Class experiment
Microscale reactions of ammonia
Try this practical to explore the reactions of ammonia with indicator solution, copper(II) sulfate solution and Nessler’s reagent. Includes kit list and safety instructions.
- Class experiment
Measuring density
By measuring the relative mass of seawater and tap water, students will be able to discover the density of these liquids. Includes kit list and safety instructions.
- Class experiment
The chemistry of thiosulfate ions
Sodium thiosulfate has several interesting reactions with a variety of chemicals. This experiment will let students explore and record these reactions. Includes kit list and safety instructions.
- Class experiment
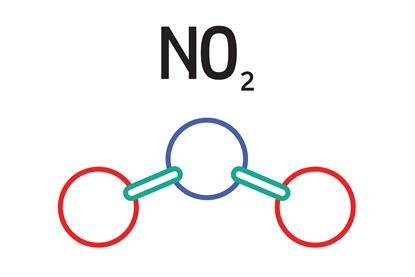
Some reactions of nitrogen dioxide
Using a range of chemicals and solutions, students can create an experiment that will explore some of the reactions of nitrogen dioxide. Includes kit list and safety instructions.
- Class experiment
Testing acids and bases on a microscale
Test various substances with indicator solution and look for colour changes in this microscale class practical. Includes kit list and safety instructions.
- Class experiment
Mass changes in chemical reactions
Perform two chemical reactions to see whether any mass changes occur in this microscale class practical. Includes kit list and safety instructions.
- Class experiment
The oxidation of cyclohexanol by nitric acid
Perform a ring opening oxidation using nitric acid to produce the dicarboxylic acid, 1,6-hexanedioic acid (adipic acid) – and then use the solid crystals that form to determine a melting point. Includes kit list and safety instructions.
- Class experiment
Exploring the chemistry of chromium, molybdenum and tungsten
Discover how transition elements differ in aspects of colour, precipitate formation, changes in oxidation state and equilibria. Includes kit list and safety instructions.
- Class experiment
Brady’s test for aldehydes and ketones
Identify aldehydes and ketones using Brady’s reagent (2,4-dinitrophenylhydrazine) in this microscale experiment. Includes kit list and safety instructions.
- Class experiment
The chemical properties of phenol
Observe and interpret some of the chemical reactions of hydroxybenzene (phenol), by adding five different substances to a Petri dish, and noting down findings. Includes kit list and safety instructions.
- Class experiment
Preparing ethyne on a microscale
Generate ethyne gas with calcium carbide and test its properties in this microscale class practical. Includes kit list and safety instructions.
- Class experiment
Observing chemical changes
Try this microscale practical to explore the chemical changes in displacement, redox and precipitation reactions. Includes kit list and safety instructions.
- Class experiment
Diffusion of gases and relative molecular mass
Try this class practical to explore the diffusion of gases and how relative molecular mass affects rate of diffusion. Includes kit list and safety instructions.
- Class experiment
Redox chemistry with dichromate ions
Observe the colour changes that occur with the reduction of dichromate ions by hydrogen peroxide. Includes kit list and safety instructions.
- Class experiment
Oxidation states of iron
Compare the two main oxidation states of iron and consider explanations for differences in this microscale practical. Includes kit list and safety instructions.
- Class experiment
Microscale reactions of metals with acids
Try this class practical to explore reactivity series with various metals as they react with acids on a microscale. Includes kit list and safety instructions.
- Class experiment
Unsaturation test with potassium manganate(VII)
Use a solution of potassium manganate to test for unsaturation in organic compounds in this microscale practical. Includes kit list and safety instructions.
- Class experiment
Properties of group 2 elements
Microscale experiment where various anion solutions are added to drops of group 2 element cations. Includes kit list and safety instructions.
- Class experiment
Testing for unsaturation with bromine on a microscale
Try this class experiment to prepare elemental bromine and use it to test for unsaturation in organic compounds. Includes kit list and safety instructions.
- Class experiment
Oxygen and methylene blue
Reacting hydrogen peroxide, and potassium manganate together will produce detectable oxygen so by using methylene blue solution, and a gas generating apparatus students can test for the presence of oxygen in this practical. Includes kit list and safety instruction.
- Class experiment
Synthesis of aspirin on a microscale
Use this class practical to produce aspirin in a microscale esterification reaction using phosphoric acid as a catalyst. Includes kit list and safety instructions.
- Class experiment
Energy changes in neutralisation
Study energy changes in two chemical reactions using thermometer strips to measure temperature in this experiment. Includes kit list and safety instructions.
- Class experiment
Formation of TCP (2,4,6-trichlorohydroxybenzene)
Delve into preparing TCP by reacting hydroxybenzene (phenol) with chlorine gas, and create this distinctive smelling compound.
- Class experiment
Investigating redox reactions on a microscale
Carry out two redox reactions and observe and interpret the results in this microscale class practical. Includes kit list and safety instructions.
- Class experiment
The microscale synthesis of indigo dye
Carry out a microscale organic synthesis, the result of which will leave students with indigo dye. Includes kit list and safety instructions.
- Class experiment
The treatment of oil spills
Tackle the real-life environment problem of oil spills in your classroom, by creating and then treating a micro version of an oil event. Includes kit list and safety instructions.
- Class experiment
Some reactions of carbon dioxide
Create carbon dioxide from marble chips and acid, then test for its reaction with barium hydroxide by observing the carbonate precipitate. Includes kit list and safety instructions.
- Class experiment
The microscale synthesis of azo dyes
Synthesise an azo dye, and use it to change the colour of cotton, with this class experiment. Includes kit list and safety instructions.
- Class experiment
Sulfate and carbonate solubility of Groups 1 and 2
Try this microscale practical to explore the properties of elements in Groups 1 and 2 as they form various precipitates. Includes kit list and safety instructions.
- Class experiment
Exploring the properties of the carvones
Test the smell of each enantiomer of carvone and detect the differences
- Class experiment
Measuring the amount of vitamin C in fruit juices
Explore ascorbic acid in fruit juices through titration in this experiment, with specimen results and calculations, stock solutions, and detailed notes included.
- Class experiment
Displacement reactions of metals on a microscale
Examine the reactions between various metals and metal salt solutions in this microscale class practical. Includes kit list and safety instructions.
- Class experiment
Electrolysis using a microscale Hoffman apparatus
Investigate the electrolysis of sodium sulfate solution using a microscale Hoffman apparatus in this class practical. Includes kit list and safety instructions.
- Class experiment
The chemistry of silver
Discover the properties of silver compounds with redox reactions, complex formation and colour/state changes. Includes kit list and safety instructions.
- Class experiment
Analysis of aspirin tablets on a microscale
Try this microscale class practical to analyse aspirin tablets and find out how much salicylic acid is present. Includes kit list and safety instructions.
- Class experiment
The temperature changes induced by evaporation
Explore the rate of evaporation for a trio of liquids, using just a temperature strip, and our worksheet. Includes kit list and safety instructions.
- Class experiment
Properties of stereoisomers
By soaking cotton wool in two limonene enantiomers, and adding a stereoisomer, students can explore the differences between each chemical and discuss how they each might react in different conditions. Includes kit list and safety instructions.
- Class experiment
Using a microscale conductivity meter
Explore electrical conductivity with this practical that allows students to test different materials for how well a current will pass through them. Includes kit list and safety instructions.
- Demonstration
Turning copper coins into ‘silver’ and ‘gold’
Perform what looks like alchemy with ordinary copper coins in this teacher demonstration. Includes kit list and safety instructions.
- Class experiment
The effect of temperature on solubility
Hot or cold, which water is better for soluble substances? Explore your finding from this practical into the effect of temperature on solubility. Includes kit list and safety instructions.
- Class experiment
The reactivity of the group 2 metals
Compare group 1 and group 2 metals with this practical that shows their reactivity rates, where students can take control of their own observations and come to their own conclusions
- Class experiment
Producing a foam
Explore foams and their properties in this experiment, so students learn how foam is produced and produce their own. Includes kit list and safety instructions.
- Class experiment
Electricity from chemicals
Use various metals, in pairs, and n electrolyte to form a cell. Then observe the formation of ions around the reactive metal, and compare the speed with which they form around the less reactive metal. Includes kit list and safety instructions.
- Class experiment
The electrolysis of solutions
Electricity is passed through various solutions and the products are identified. Includes kit list and safety instructions
- Class experiment
The volume of 1 mole of hydrogen gas
Understand the volume of one mole of hydrogen gas through a magnesium and acid reaction, taking note of the temperature and pressure. Includes kit list and safety instructions.
- Class experiment
The effect of temperature on reaction rate
Discover more about collision theory in this practical, where a sodium thiosulfate and hydrochloric acid mixture produce an interesting reaction. Includes kit list and safety instructions.
- Class experiment
The effect of concentration and temperature on reaction rate
Reaction rate can be altered by many things, in this practical students explore how temperature and concentration effect reaction in an closer look at kinetics. Includes kit list and safety instructions.