Dry ice sublimes, as do iodine and mothballs. This experiment involves the study of another common substance that sublimes – air freshener
Pieces of solid air freshener are heated in a hot water bath and the vapour caught by cooling with ice. No liquid will be observed so students will be able to appreciate that a solid has turned directly to a gas without passing through the liquid phase. A fume cupboard, or other method of preventing escape to the air, is required for this experiment.
This experiment is best done as a demonstration. It takes a while for anything to happen, so learners can work through questions 1–5 in the student worksheet once you have set up the demonstration. Once learners have completed the questions, they can go over to the demonstration in small groups to make their observations. Learners can observe the demonstration periodically during the lesson to record changes over time.
Most of the substances in the air fresheners are harmful. This is not a problem in day-to-day use as the vapour pressure and hence the amount which is in the air is low. However, heating them causes them to sublime quickly and they could reach harmful levels in the air so a fume cupboard or other method of preventing escape to the air is necessary.
Teaching notes
Sublimation is the vaporisation of a solid. The opposite process – the formation of a solid directly from a vapour – is called deposition. The heat from the water bath causes the solid air freshener to sublime. The cold beaker causes the vaporised air freshener to re-form the solid. If there is space in the fume cupboard, leave a few lumps of solid air freshener to sublime at room temperature (not in a hot water bath) to demonstrate the difference in rate of change.
Sublimation is caused by the absorption of heat, which provides enough energy for some molecules to overcome the attractive forces of their neighbours and escape into the gas phase. Learners will be less familiar with this process as normally we expect a solid to melt into a liquid before boiling or evaporating into a gas. However, sometimes conditions such as vapour pressure and temperature mean that the liquid phase is skipped.
If possible, use a coloured air freshener and notice that the material that collects on the cold beaker is white. The dye does not sublime because it is not chemically a part of the compound that does sublime. Vapour deposition is an important industrial process for separation and purification.
You can use other materials that sublime including iodine, naphthalene and dry ice (carbon dioxide), see below for more details.
Technical notes
Equipment required for a teacher demonstration
- Eye protection
- Access to a fume cupboard
- Gloves (for those with sensitive skin)
- Beakers (100 cm3), x2
- Stand, boss and clamp
- Shallow dish
- Thermometer (-10–110 °C)
- Kettle (for hot water)
- Solid air freshener (HARMFUL), a few lumps
Gel-type air fresheners will not work.
Safety and hazards
- Read our standard health & safety guidance
- Wear eye protection and gloves. Work in a fume cupboard.
- Air freshener – solid toilet bowl cleaners work best; if possible use a coloured one. If cheap ones containing 1,4-dichlorobenzene (para-dichlorobenzene), C6H4Cl2(s), are used, handle them with tongs in a fume cupboard. Para-dichlorobenzene is HARMFUL and DANGEROUS FOR THE ENVIRONMENT – see CLEAPSS Hazcard HC023.
Alternative materials
Please note the following when using another material to do this demonstration:
- Iodine (HARMFUL, DANGEROUS FOR THE ENVIRONMENT): use only a few crystals and do the activity in a fume cupboard – see CLEAPSS Hazcard HC054. See also Sublimation of iodine (Exhibition chemistry).
- Naphthalene (HARMFUL, DANGEROUS FOR THE ENVIRONMENT): do the activity in a fume cupboard. Naphthalene mothballs must be heated to near 70°C to sublime – see CLEAPSS Hazcard HC046b.
- Dry ice sublimes at –78.5°C and above. Handle with tongs or thermal gloves. You will not be able to watch the solid re-form but it is great for observing the change from solid to gas – see CLEAPSS Hazcard HC020a.
Procedure
- Wear eye protection and work in a fume cupboard.
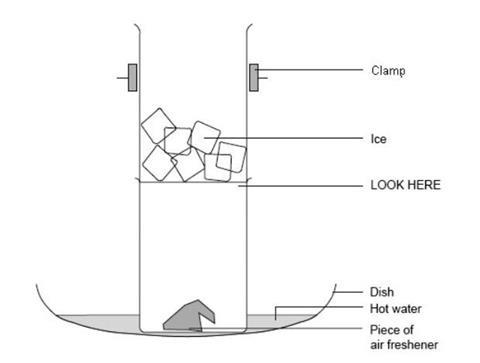
- Place a few lumps of air freshener in the bottom of one of the 100 cm3 beakers.
- Fill the other beaker three-quarters full of ice.
- Stand the beaker containing the air freshener in a shallow dish.
- Carefully, clamp the beaker containing the ice in position on top of the beaker of air freshener. See diagram.
- One-third fill the shallow dish with warm water (hotter than 45°C).
- Observe what happens to the solid. Be patient as it may take a while.
Downloads
Sublimation of air freshener unscaffolded student sheet
Handout | PDF, Size 0.2 mbSublimation of air freshener scaffolded student sheet
Handout | PDF, Size 0.2 mbSublimation of air freshener teacher notes
Handout | PDF, Size 0.33 mbSublimation of air freshener unscaffolded student sheet
Editable handout | Word, Size 0.48 mbSublimation of air freshener scaffolded student sheet
Editable handout | Word, Size 0.49 mbSublimation of air freshener teacher notes
Editable handout | Word, Size 0.53 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. Updated in 2024 with additional student questions and activities added by Dorothy Warren.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry






































No comments yet