Keep on top of risk assessments to ensure your chemistry practicals are safe
Practical work is one of the highlights of many students’ chemistry education. Key to ensuring that everyone remains safe during practical work are the risk assessments we carry out, both before and during the activities. Risk assessment can sometimes have a bad name, perhaps arising from overly bureaucratic processes leading to paperwork that is hard to understand or rarely used. Equally, to some, health and safety can seem to stand in the way of the activities that we want to use with our students.
Good risk assessment is a process that identifies hazards, assesses risks and reduces those risks with control measures. We assess risk all the time. When we walk down the street, should we cross the road at this busy junction or walk 20 metres further to the traffic lights? Given our use of hazardous chemicals, apparatus and techniques in our chemistry lessons, we should expect nothing less of ourselves than carrying out good risk assessment.
Our legal responsibility
The Health and Safety at Work Act (1974) and subsequent regulations, such as the Control of Substances Hazardous to Health, are the legal framework that we work under. These laws and regulations place duties on both our employers and on us as employees. Employers must provide healthy and safe working conditions for employees and others (eg students), and provide policy, information and training on health and safety. This includes risk assessing the activities that we carry out in our work. We, as employees, must take reasonable care of our own and others’ safety, and cooperate with our employer.
Experience and common sense are important in identifying hazards, making technicians and more experienced teachers a valuable source of advice
In practice, we would never expect our head teacher to personally risk assess the individual activities we use. Instead, the school will provide access to expert model risk assessments and advice from organisations such as CLEAPSS (in England, Wales and Northern Ireland) and SSERC (in Scotland). We are expected, as employees, to adapt these model risk assessments to our circumstances. Responsibility is also delegated to heads of departments to ensure the implementation of appropriate practice.
In practice, we would never expect our head teacher to personally risk assess the individual activities we use. Instead, the school will provide access to expert model risk assessments and advice from organisations such as CLEAPSS (in England, Wales and Northern Ireland) and SSERC (in Scotland). We are expected, as employees, to adapt these model risk assessments to our circumstances. Responsibility is also delegated to heads of departments to ensure the implementation of appropriate practice.
The process of risk assessment can be summarised in three steps:
- Identify the hazards
- Assess the risks
- Reduce the risks with control measures
Hazards can come in the form of the chemicals being used (and produced), the apparatus involved and the procedures being carried out. All hazardous chemicals come with warning symbols indicating whether the chemical is toxic, corrosive, irritant etc. Apparatus hazards can include sharp edges (scalpels, glassware etc), extreme temperatures (Bunsen burners, ice etc) and electrical circuits (balances, water baths etc). Experience and common sense are important in identifying hazards, making technicians and more experienced teachers a valuable source of advice.
Risk has two related factors. First, what is the likelihood of any harm occurring, and second, how severe will that harm be? A spill of acid is much more likely with a group of 11 year-olds than a post-16 group, as the latter have more experience and manual dexterity. Remember, our students are one of the biggest sources of risk in our lessons. If a spill does occur, skin contact with 0.1 M hydrochloric acid is much less severe than contact with concentrated hydrochloric acid.
Reduce the risks
Following identification of the hazards and risks, our responsibility is to reduce those risks to the lowest level practicable by implementing control measures. Control measures are steps we take to reduce the harm caused if something goes wrong. Importantly, these control measures need to be proportionate to the risks involved. Lack of proportionality can lead to many of the health and safety myths much loved by newspaper headline writers. Think of the school making students wear goggles for their conker fights.
Following identification of the hazards and risks, our responsibility is to reduce those risks to the lowest level practicable by implementing control measures. Control measures are steps we take to reduce the harm caused if something goes wrong. Importantly, these control measures need to be proportionate to the risks involved. Lack of proportionality can lead to many of the health and safety myths much loved by newspaper headline writers. Think of the school making students wear goggles for their conker fights (bit.ly/3iQ6mkj).
A wide range of control measures are available and their general effectiveness decreases as you go down the hierarchy of controls pyramid.
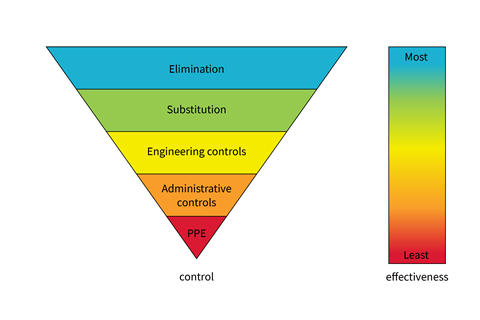
Elimination is the most effective control measure. If the hazard is not present, it cannot cause harm. In schools, benzene is not recommended because it is carcinogenic. As petrol is a formulation containing approximately 1% benzene, we don’t use petrol in school science either. However, toxic chemicals such as sulfur dioxide and chlorine can be used, or made, assuming good control measures are put in place. For example, using a fume cupboard or reducing the scale of the experiment. You can learn more about this in the book I wrote with Bob Worley, Understanding chemistry through microscale practical work.
Elimination is the most effective control measure. If the hazard is not present, it cannot cause harm. In schools, benzene is not recommended because it is carcinogenic. As petrol is a formulation containing approximately 1% benzene, we don’t use petrol in school science either. However, toxic chemicals such as sulfur dioxide and chlorine can be used, or made, assuming good control measures are put in place. For example, using a fume cupboard or reducing the scale of the experiment through microscale practicals (rsc.li/3NNtPkH). You can learn more about this in the book I wrote with Bob Worley, Understanding chemistry through microscale practical work: bit.ly/3NuIZuK
Substitution is the next most effective control measure. The simplest form of substitution is using the lowest concentration of a substance necessary to produce the required outcome. For example, many common reactions with hydrochloric acid in pre-16 practicals work fine with a 0.4 M solution. Similarly, hazardous substances, such as phenol, can be effectively substituted with safer alternatives, like methyl 4-hydroxybenzoate.
Fume cupboards, as mentioned above, are a type of engineering control. Safety screens are also common, protecting students and adults when explosive reactions are carried out. Showing the reactivity of group 1 metals with water provides a good example.
Fume cupboards, as mentioned above, are a type of engineering control. Safety screens are also common, protecting students and adults when explosive reactions are carried out. Showing the reactivity of group 1 metals with water (rsc.li/3DpzEQq) provides a good example.
Administrative controls can be thought of as ways of working. For example, how do we train our students to think about their own and others’ safety in the laboratory; how do they move around the space, how do they access apparatus and materials?
Personal protective equipment (PPE) is classed as the least effective method of reducing risk. Eye protection is the most used PPE. Safety spectacles are fine for most activities. Splashproof goggles provide increased protection and are necessary when using some chemicals. However, they are less comfortable to wear and prone to misting. This tends to lead to students removing their goggles more often, making them less useful as a control measure.
Model risk assessments and procedures
The CLEAPSS and SSERC model risk assessments come in a variety of forms. For example, CLEAPSS hazcards and the SSERC hazardous chemicals database provide detailed information about the various forms of many common substances used in practical work. The CLEAPSS practical procedures include details of how to successfully carry out activities. Practical instructions, videos and resources are also available on the RSC Education Practical resources page. The Preparing a salt practical video is a great resource to highlight risks, hazards and control measures to your 14–16 students. The supporting resources include a risk assessment for learners to complete, too.
Model risk assessments and procedures
The CLEAPSS and SSERC model risk assessments come in a variety of forms. For example, CLEAPSS hazcards (bit.ly/3uDtloi) and the SSERC hazardous chemicals database (bit.ly/3tSpBAc) provide detailed information about the various forms of many common substances used in practical work. The CLEAPSS practical procedures (bit.ly/3DHEWXD) include details of how to successfully carry out activities. Practical instructions, videos and resources are also available on the RSC Education Practical resources web page (rsc.li/3IPlzNj).
In summary, risk assessment is the process that we use to minimise the risks we are all exposed to while allowing students to learn and experience the wonders of chemistry. Model risk assessments give us the professional guidance to start from. We then adapt them to take account of our particular circumstances. During practical experiments, we continue to assess risks. If things aren’t working, or behaviour isn’t appropriate, then the safest step can be to abandon the activity, trying again another time. Risk assessments allow us to do our jobs both safely and professionally.
David Paterson is a chemistry teacher at Aldenham School and chemistry adviser at CLEAPSS














No comments yet