The general thaw in East/West relations and substantial government cut-backs in MI6 expenditure has resulted in people on cultural exchanges being used to carry sensitive diplomatic messages
Development of new techniques using invisible ink have become essential as messengers using other techniques have mysteriously disappeared!
When dry, the paper must be sent to MI6 together with instructions and materials for ‘developing’ the message. You are permitted 7 sheets of paper. You may send more than one message.
This experiment should take 60 minutes.
Equipment
- Eye protection
- Suggest students are warned in advance to bring an old shirt or a CDT apron to the session
- Cocktail sticks or empty old fountain pens or cotton swabs
- Beakers
- Test tubes
- Pink paper
- White paper.
- Sulfuric acid
- Nitric acid
- Lemon juice
- White vinegar
- Cobalt chloride
- Phenolphthalein indicator solution
- Sodium hydroxide
- Potassium thiocyanate solution
- Iron(lll) chloride solution
Health, safety and technical notes
- Read our standard health and safety guidance here.
- Wear eye and clothing protection.
- This is an open-ended problem-solving activity, so the guidance given here is necessarily incomplete.
- Care must be taken when developing those inks that require a heat source. (The ink should singe before the paper does.)
Suggested concentrations:
- Sulfuric acid, 0.5 M H2SO4(aq), is of low hazard. See CLEAPSS Hazcard HC098a.
- Nitric acid, 0.4 M HNO3 (aq), is an IRRITANT. See CLEAPSS Hazcard HC067.
- Cobalt(II) chloride-6-water, 0.1 M CoCl2.6H2O (s), is a carcinogen (by inhalation) mutagen, reproductive toxin, skin/respiratory sensitiser and hazardous to the aquatic environment. See CLEAPSS Hazcard HC025.
- Phenolphthalein indicator (0.1% w/v in 60% v/v ethanol) is flammable. See CLEAPSS Hazcard HC032. (If in methylated spirits rather than pure ethanol, it is also harmful if swallowed and can cause damage to organs)
- Sodium hydroxide solution, 0.5 M NaOH (aq), is corrosive to skin and eyes. See CLEAPSS Hazcard HC091a.
- Potassium thiocyanate solution, 0.1 M KSCN (aq) is an eye irritant, harmful if swallowed, inhaled or in contact with skin and releases toxic gas in contact with acids. See CLEAPSS Hazcard HC095a.
- Iron(III) Chloride, 0.5 M FeCl3, is CORROSIVE and HARMFUL if swallowed. See CLEAPSS Hazcard HC055b.
Possible approaches
Invisible inks consist of chemical solutions that are ‘colourless’ before developing, but which become visible when (i) heated, (ii) observed under ultraviolet light, or (iii) treated with other chemicals.
Invisible inks that respond to heat:
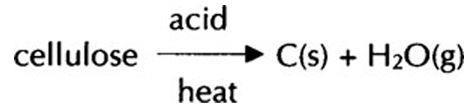
- Acids, eg sulphuric acid, nitric acid, lemon juice, white vinegar (colourless, go black with heat). Lemon juice is a very good magic ink. More concentrated acids are better than very dilute acids. NB When hot acid is concentrated, it chars paper by reacting with the cellulose to produce black carbon.
- Cobalt chloride (dilute cobalt chloride solution is light pink, almost colourless. If it is used as an ink, the “invisible” writing goes blue on warming, due to dehydration of the salt. If you then breathe on the paper the writing once more disappears).
To develop the message, slowly pass the paper above a hot light bulb (check that the lamp is safely wired and earthed). Alternatively, use a radiator or sunlight.
Invisible inks that require chemical treatment:
- Phenolphthalein indicator solution (colourless, goes pink with alkali solution).
- Potassium thiocyanate solution (colourless, goes red with iron(lll)chloride solution).
- The Fe(lll) ion reacts with the thiocyanate ion (SCN-) to produce the red complex, Fe(SCN)2+.
Possible extension
To find other ‘magic’ inks.
Notes
Any acid or alkali solutions should be neutralised with a weak acid or alkali (as appropriate) before being washed to waste.
Cobalt chloride should be collected for appropriate disposal.
Downloads
Making invisible ink
PDF, Size 27.18 kb
Additional information
The resources were originally published in the book In Search of Solution P. Borrows, K. Davies and R. Lewin, Royal Society of Chemistry, 1990.
This experiment was based on an idea contributed by J. Crellin.


















No comments yet