Everything you need to perform simple distillation with your 14–16 learners, distilled into a poster and fact sheet
Distillation is an ancient separation technique, with its roots in the preparation of alcoholic spirits (such as vodka and whisky) and pre-chemistry studies (called alchemy). A still is the apparatus used for distillation. The term distil comes from the Latin root words meaning ‘to drop down’.
Want the poster?
Make sure you come back to download your poster on 5 January. In the meantime, sign up for print issues of EiC by 8 December to receive this poster, plus another one, in the January issue.
The separation technique involves evaporating or boiling a liquid, collecting the vapour and condensing the vapour back to a liquid.
Distillation therefore combines two state changes: vaporisation (liquid to gas) and condensation (gas to liquid).
Scientists use distillation to separate liquids from mixtures with soluble and insoluble solids, and from mixtures with other liquids, for example:
- a mixture of water and sand (an insoluble solid)
- a mixture of water and sodium chloride (a soluble solid)
- a mixture of water and ethanol (a soluble liquid)
The condensed liquid you collect during distillation is called the distillate.
View and download more infographics
Did you know … ?
Part of the water cycle can be considered as distillation. The sun heats surface water and it evaporates. The vapour rises in the atmosphere, cools and condenses, then precipitates back to the surface, for example as rain.
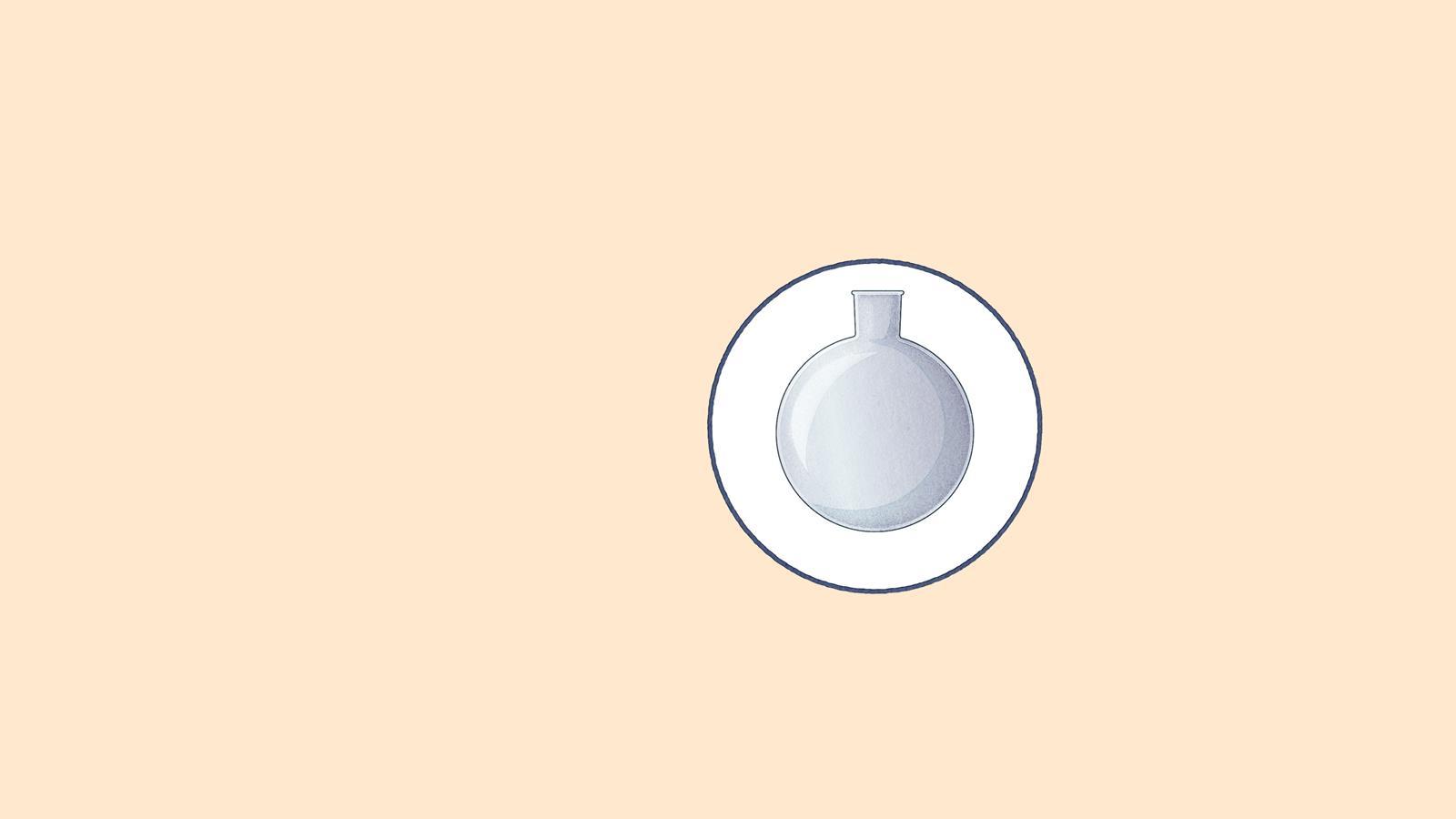


Round-bottomed flask: contains the mixture and anti-bumping granules
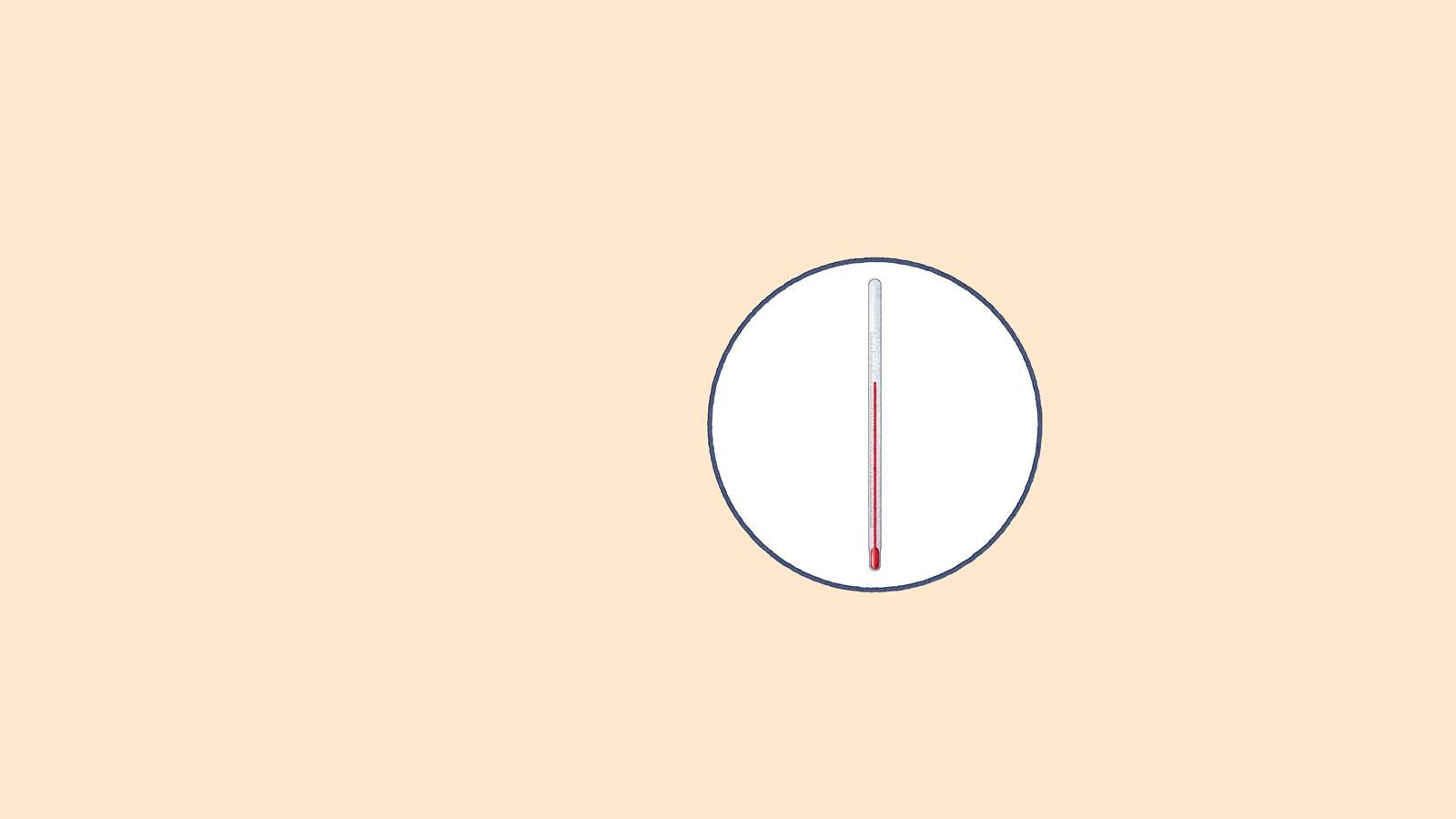
Thermometer: measures the vapour's temperature
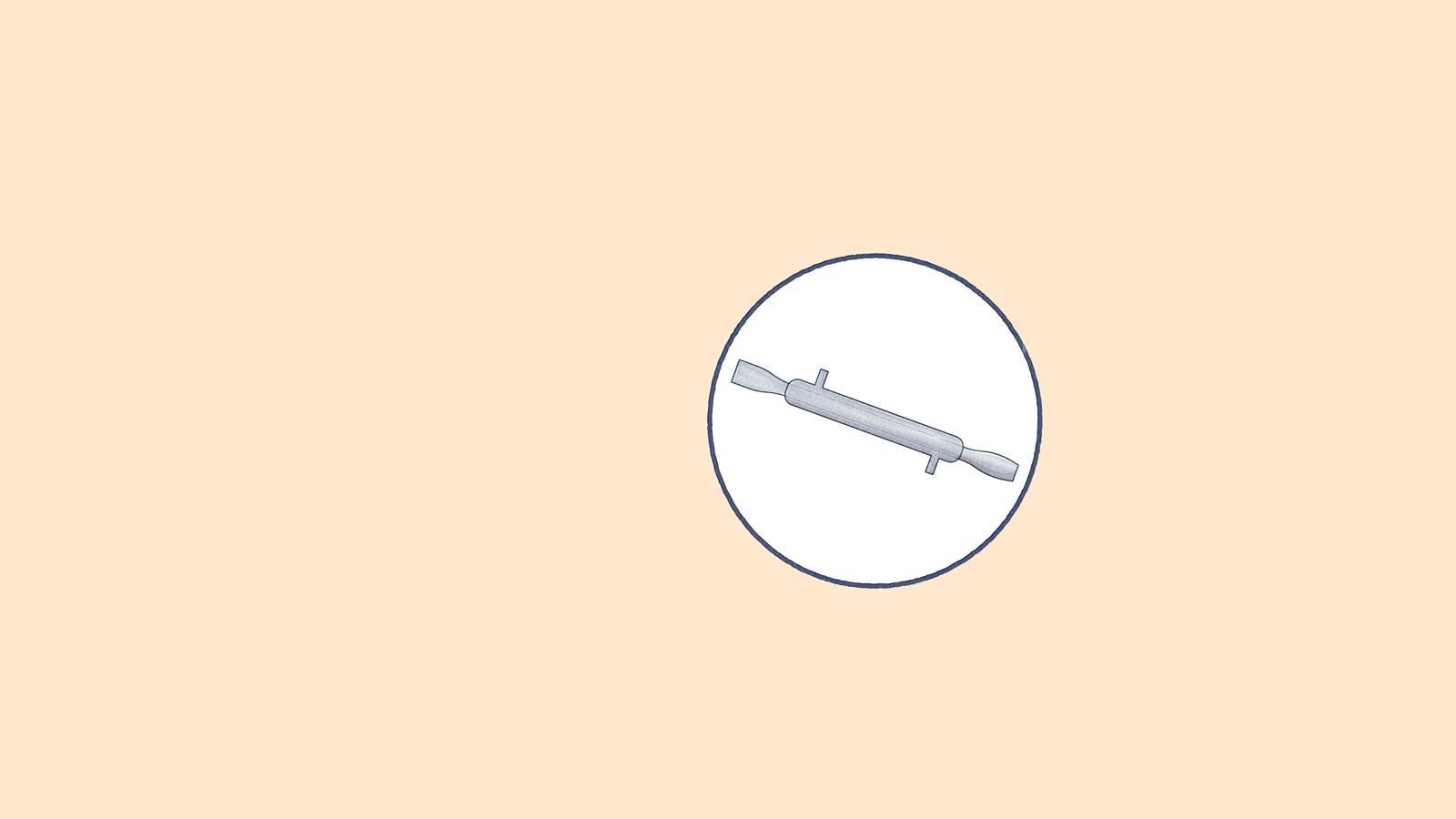
Still head: connects the flask and the condenser
Condenser: the cool water condenses the distillate vapour
Still receiver: connects the condenser to the receiving flask
Receiving flask: e.g. a beaker, round-bottomed flask or test tube
Apparatus
In a simple distillation setup you contain the mixture in a boiling tube, connected to a delivery tube by a bung. The vapour passes through the delivery tube, the air cools it, it condenses and is collected in a receiving flask.
A more sophisticated setup uses a water-cooled condenser. The most common condenser used in school chemistry laboratories is the Liebig condenser. This is made from two concentric tubes. The vapour passes through the inner tube. Cooling water passes through the outer tube. You heat the mixture in a round-bottomed (or a pear-shaped) flask which you connect to the condenser by a still head. You may use a still receiver to connect the condenser to the receiving flask. School chemistry laboratories usually use special glassware called QuickfitTM for this type of distillation.
Watch the video
Show learners this practical video demonstrating the separation of water from a coloured solution. Complement live practical work or provide an alternative route to understanding simple distillation when you don’t have access to the lab. Download the accompanying slides including instructions, learner activities and teacher and technician notes.
Heating
Distillation in the school chemistry laboratory involves actively heating the mixture.
You can provide heat with:
- a Bunsen burner or fuel burner
- a water bath – electric or Bunsen burner-heated
- a heating mantle – electric heating of a metal block
- a sand or oil bath – electric heating of sand or oil
You heat the mixture inside a glass vessel that you connect to the condensing apparatus.
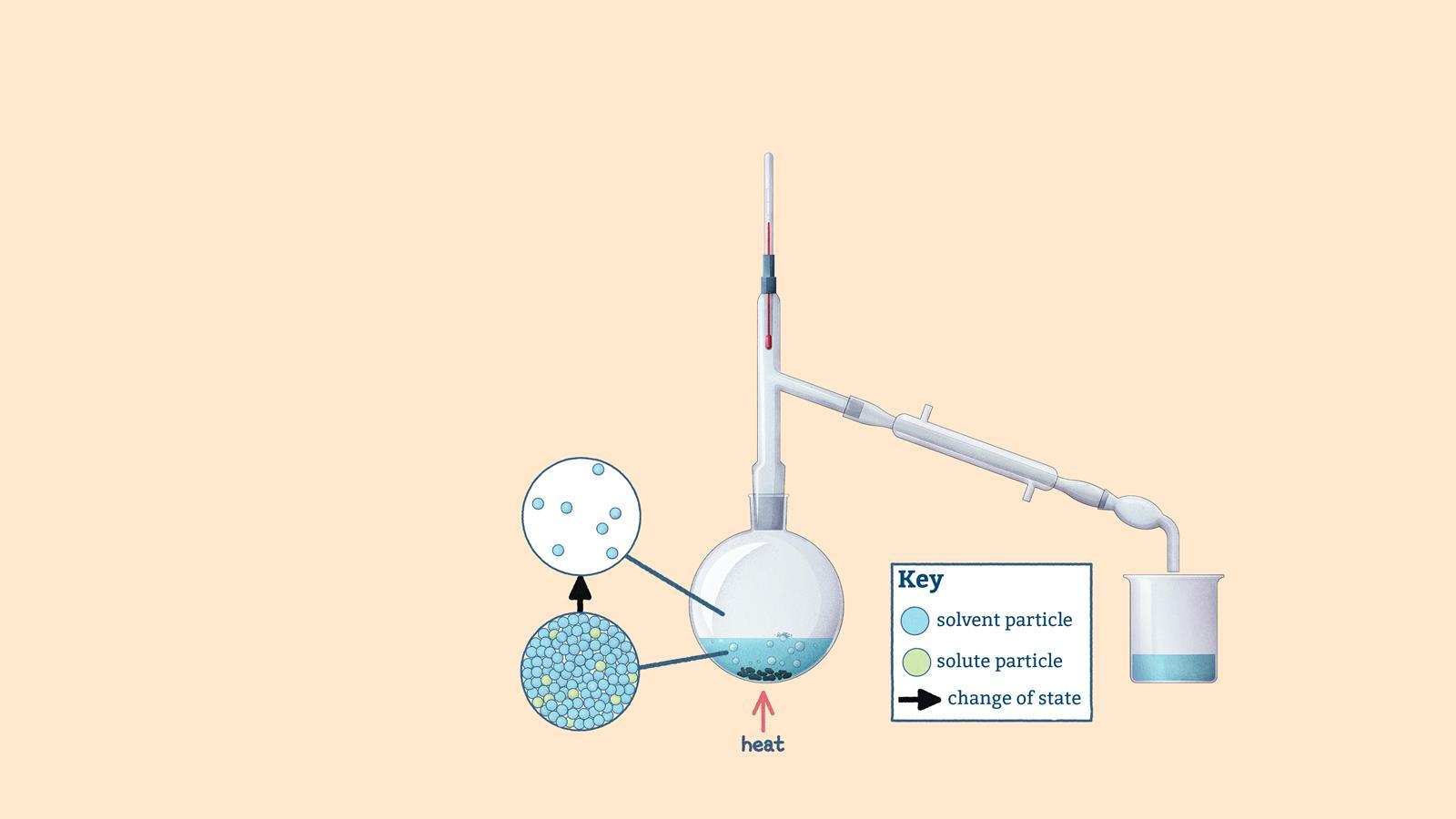




The mixture contains solvent and solute particles. Heating causes bubbles of solvent particles to form and solvent to evaporate
Solvent vapour rises up the still head, with some condensing into drops on the inside
The thermometer measures the temperature of the solvent vapour
Solvent vapour condenses into drops on the cooled tube
Drops of solvent (now distillate) collect in a receiving flask
Did you know … ?
A similar process is used in a solar still to produce water in remote areas or during emergencies. Heat from the Sun evaporates water from dirty mixtures, the vapour is trapped on a surface and the condensed water drips into a cup.
Separating liquids
Separating liquid mixtures involves selectively boiling off one of the substances in the mixture, so careful heat control is required. For good separation, the boiling points of the liquids need to be significantly different, usually by more than 100°C.
When boiling points are closer together, the distillate formed is a concentrated mixture rather than a pure substance. For example, distilling mixtures of ethanol and water produces a more concentrated ethanol solution, as ethanol has a boiling point of 78ºC and water has a boiling point of 100°C.
This is the basis of the production of alcoholic spirits. For example, fermented mixtures of barley and wheat produce an approximately 10% ethanol solution. This is then distilled to an approximately 40% ethanol solution to make whisky. Adding a fractionating column between the boiling flask and still receiver allows for improved separation of liquids with close boiling points. This separation is called fractional distillation.
Downloads
Distillation fact sheet
Handout | PDF, Size 0.12 mbDistillation fact sheet
Editable handout | Word, Size 0.44 mb














No comments yet