Experiment with plastic bottles with a mixture of hydrogen gas and air to make ‘rockets’
In this experiment, students observe as a ‘rocket’ made from a plastic fizzy drink bottle is filled with a mixture of hydrogen gas and air. The rocket is launched by igniting the mixture with an electric spark, and will fly several metres, propelled by the same reaction used to power the Space Shuttle.
Use the demonstration for open days, or to introduce ideas about the role of combustion energy changes in fuels.
The demonstration requires quite a bit of preparation (and rehearsal!) time initially but once the basic launcher set-ups have been put together, it is just a question of a supply of suitable drinks bottles – and hydrogen.
To minimize the risk of bottles bursting on launch, use only those intended for fizzy, rather than still, drinks, as they will be made to withstand the pressure. They should be made of PET and marked with recycling symbol 1.
Students, and members of the public, who may be alarmed by explosions should be warned. Those close by should be advised to put their fingers in their ears in case a particularly loud explosion occurs.
The time taken for the demonstration should be about 5–10 minutes depending on how much preparation is done beforehand, such as filling the bottle(s).
Equipment
Apparatus
- Eye protection for demonstrator
- Ear protection for demonstrator
- Safety screens
- Plastic fizzy drink bottles, 500 cm3 (or smaller if done indoors), x2–3 (see note 8 below)
- Rubber bungs, to fit bottle, x2 (see note 4)
- Plastic bowl, for filling bottle with gas over water
- Piezoelectric gas lighter (or 0–5 kV EHT supply) (see note 5)
- Leads, about 1 m, fitted with crocodile clips, x2
Optional
- Piece of softwood, about 15 x 2 x 2 cm (see note 6)
- Two small screw eyes (see figure 3)
- Strong rubber bands, to fit tightly around body of bottle, x2
- String, about 10 m
- Plastic drainpipe, diameter larger than that of drink bottle, about 30 cm long (see note 7)
Chemicals
- Access to a hydrogen (EXTREMELY FLAMMABLE) cylinder fitted with a regulator and a length of rubber tubing
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye and ear protection throughout.
- Hydrogen, H2(g), (EXTREMELY FLAMMALBE) – see CLEAPSS Hazcard HC048.
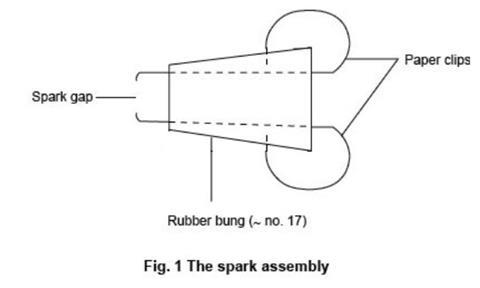
- Construct the spark assembly as follows. Make two holes about 5 mm apart in one of the rubber bungs by straightening a paper clip and, holding it with pliers, heating it in a Bunsen flame before pushing it through the bung. Insert a straightened paper clip through each hole so that about 5 mm protrudes from the narrow side of the bung. Bend back the other ends of the paper clips and push them into the side of the bung to stop them rotating – see figure 1 below.
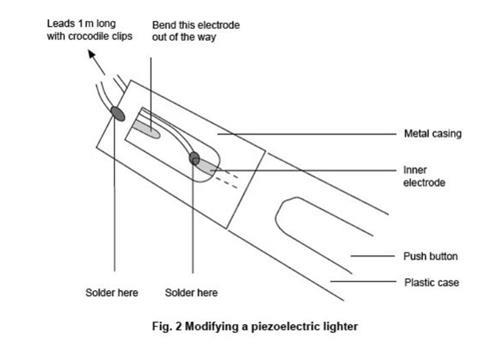
- Construct a spark generator as follows. Take a piezoelectric gas lighter (obtainable from hardware shops – NOT the type with a built-in gas supply ignited by the spark) and solder a pair of insulated leads about 1 m long to the two spark terminals. Bend the terminals away from each other so that the lighter does not spark across the original terminals – see figure 2. Fit crocodile clips to the other ends of the leads and attach them to the spark assembly. Press the spark assembly firmly into the mouth of the bottle and check that it is still sparking reliably inside the bottle. Alternatively connect the EHT supply to the spark assembly using the insulated leads, and check the voltage setting required to produce a spark reliably.
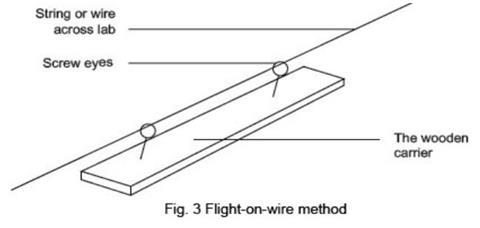
- For the ‘flight-on-wire’ method screw the eyes into the piece of wood, about 2 cm from each end. Thread the string through the screw eyes. Run the string across the room at a suitable height to avoid any obstructions, attaching it firmly at both ends. One end must allow the spark assembly to be clamped close to the string. If the string slopes gently upwards, it will help to slow the rocket down. Place safety screens on either side of the assembly, to protect audience and demonstrator.
- For the drainpipe method clamp the drainpipe securely so that the rocket will be launched safely away from the audience and any obstructions. Bear in mind it will fly several metres (a 500 cm3 bottle can travel up to 30 m). It is preferable to do the launch outside, unless using very small bottles. It will be necessary to clamp the spark assembly at the base of the launch tube. Place safety screens on either side of the assembly, to protect audience and demonstrator.
- If repeating the demonstration, check the bottle carefully for damage. Preferably use a new bottle.
Procedure
- Fill the plastic bottle with water, invert it over a bowl of water and fill it just over a quarter full of hydrogen from the cylinder (2:5 is the stoichiometric ratio for hydrogen and air by volume). It will help to have previously marked the bottle at the correct level with a waterproof marker pen.
- Keeping the bottle upright, mouth downwards, lift it slowly from the water to allow air to replace the remaining water in the bottle. Seal the bottle with a bung and carry it to the launch point.
- Replace the bung with the spark assembly. Either attach the bottle firmly to the wooden carrier using the rubber bands, or position the bottle in the launch tube. Make sure that the spark assembly is clamped firmly – see figure 4 below. If a retort stand is used, it will need to weighted down or attached to the bench with a G-clamp.
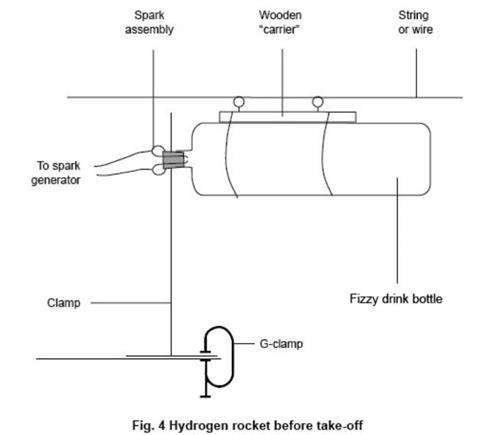
- Check the safety screens are in place and fire the rocket by operating the sparking device.
Teaching notes
Point out that the bottle is covered inside with condensation after firing, as a result of the water vapour produced by the combustion of hydrogen – not to be confused with any water that may have been left in the bottle after filling. The equation for the reaction is:
2H2(g) + O2(g) → 2H2O(g), ΔHo = –484 kJ mol–1
The energy of the reaction is released as heat, light and sound, with the rapidly expanding hot gas providing the kinetic energy to propel the rocket.
This is also the reaction used to power the Space Shuttle, after the solid boosters have burnt out, except that here the oxidant is present as part of air whereas on the Shuttle it was carried as liquid oxygen. It was the uncontrolled explosion of this mixture that led to the tragic destruction of the Space Shuttle ‘Challenger’ in 1986 with all on board, including the first teacher in space.
Hydrogen can also be used as fuel in the internal combustion engine, to power motor vehicles, with the added advantage that the only ‘exhaust gas’ is water vapour. More recently interest has turned to fuel cells, which convert hydrogen and oxygen directly into electricity. In this way the energy from the reaction can be more efficiently used to propel a vehicle than if the hydrogen is burnt. In both cases the cheap generation and safe storage of hydrogen as a fuel remain obstacles to the wider use of hydrogen power for transport.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet