Work collaboratively to build four large-scale Born–Haber cycles with this toolkit of arrow cards
This resource accompanies the infographic poster Mastering the Born–Haber cycle in Education in Chemistry which can be displayed in your classroom to reinforce learning on this topic.
Learning objectives
- Construct Born–Haber cycles involving singly and doubly charged cations and anions.
- Compare the relative sizes of the enthalpy changes involved in different Born–Haber cycles.
- Explain the relative magnitudes of enthalpy changes involving transferring electrons from and to atoms and ions.
- Calculate an unknown enthalpy change using Hess’s law, given suitable data about the other enthalpy changes in a Born–Haber cycle.
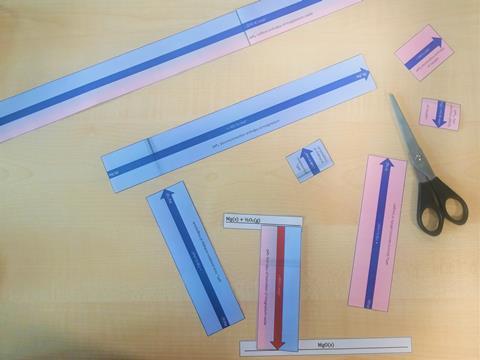
The resource contains a set of cut-out arrows drawn to scale to represent the component enthalpy changes that make up Born–Haber cycles for the formation of NaCl(s), MgCl2(s), Na2O(s) and MgO(s) from their elements. The set allows students to easily arrange the enthalpy changes of the different components as a hands-on activity to help them become familiar with the different cycles. They will need to place the arrows pointing up or down depending on whether the given enthalpy change is exothermic or endothermic, allowing them to think about the energy transfers associated with each component.
This activity can be done individually, but works well with students working in small groups or pairs to promote learning through trial, error and discussion.
Printing tips
The arrows in the toolkit need to be printed at the correct scale for the activity to work. Ensure that the printer chooses ‘paper source by page size’ and do not allow any shrinking of oversized pages to ensure the toolkit is printed ‘actual size’. You should be able to change these options in your printer settings.
Instructions for teachers
Materials
You will need:
- Three sheets of A4 stiff paper or card.
- One sheet of A3 stiff paper or card.
- Access to a laminator.
- Clear sticky tape.
- Plastic wallet or similar to store the sets of cards.
Preparation
- Print out the three A4 sheets, plus the one A3 sheet in the separate toolkit on card. The first A4 sheet contains all the components needed for NaCl. The other three cycles are made by adding the cards from the other two A4 sheets and the A3 sheet. Make sure that the printer does not rescale the pages to keep the lengths correct.
- Cut out the individual rectangular cards, taking care not to lose any small pieces. If a laminator is available, you can use this to make the cards more durable. The two halves of the large lattice enthalpies on the A3 sheet will need joining with clear sticky tape to form a hinge so they can be easily folded.
- Sort and store the cards in a plastic wallet. The cards are shaded in colours to help with sorting. If a colour printer is not available, use coloured stickers.
Suggested activity and teaching points

- Introduce Born–Haber cycles by first reviewing the enthalpy level diagram for the formation of a simple covalent compound. Remind students of the need for bond breaking (endothermic), followed by bond making (exothermic).
- Show students the equations for the enthalpy changes involved in Born–Haber cycles: enthalpy of atomisation, ionisation enthalpy, electron affinity and lattice enthalpy, distinguishing bond breaking/making from electron transfer processes.
- Give each group the set of cards needed to make NaCl. Allow them to try to construct the cycle, giving hints if necessary.
- Review the completed cycle for NaCl. Note that cards showing the formulas of the intermediate atoms and ions have not been included in the kit to reduce the number of small pieces of card. However, students could be encouraged to add them on paper once they have completed the cycle.
- Before they move on, highlight the following features of the NaCl cycle.
(a) Show how the value of the enthalpy change for any of the individual components can be calculated if the others are known. The cards give a helpful visual picture of the addition and subtraction needed.
(b) Show how the cycle can be used to compare the relative contribution of the different components to give the overall DHf of the ionic solid.
(c) Able students might also benefit by reflecting on the statement that ‘sodium atoms want to lose their outer electron’, which is a common misconception arising from the simple pre-16 models used for bonding (see question 5 on the student sheet). - Once the groups are familiar with the NaCl cycle, they can go on to attempt any or all of MgCl2, Na2O and MgO. Place the additional cards needed in separate pots around the room, so students have to think about what they need. These will introduce the additional features of the more complex cycles.
(a) The second ionisation enthalpy for doubly charged metal ions.
(b) The need to double the atomisation and electron affinity/first ionisation enthalpy for the singly charged ions combining with doubly charged ions.
(c) The large endothermic second electron affinity for doubly charged non-metal anions.
The very large arrows needed for the lattice enthalpies in these cycles are quite cumbersome but dramatically illustrate the effect of ion charge on the strength of the ionic bonding in the lattice.
More resources
- Stimulate and challenge your students with these sudoku-style puzzles, including a puzzle for the Born–Haber cycle for magnesium chloride and the Born–Haber cycle for sodium chloride.
- Discover more ideas about how to teach energy and change post-16 with this CPD article.
- Test your learners’ understanding of thermodynamics with this Starters for 10 resource, which includes questions about Born–Haber cycles.



















No comments yet