Try this simple practical to show that seawater contains a mixture of different salts
Seawater is often called salt water but it contains various different salts. In this experiment, students separate some of these salts from the mixture.
This simple experiment could be carried out early in a science course while using scientific apparatus is still a novelty, because it provides practice at using a variety of equipment and also introduces the concept of a mixture and some simple chemical tests. It can also be used to reinforce important safety messages (eg wearing eye protection), and in conjunction with Earth science topics linked to the conveyance of mineral salts into the sea via rivers.
The experiment can be carried by groups of two or three and will take about one hour to complete.
Try these related experiments
Explore this topic further by analysing the dissolved solids in seawater (a demonstration suitable for 14–16 year old students), or comparing the substances dissolved in tap water and seawater (a class practical and demonstration suitable for 11–14 and 14–16 year old students).
Equipment
Apparatus
- Eye protection
- Beaker, 250 cm3
- Beaker, 100 cm3
- Conical flask, 100 cm3
- Evaporating basin
- Filter funnel
- Filter paper
- Bunsen burner
- Heat resistant mat
- Tripod
- Gauze
- Teat pipette
Chemicals
- Seawater, 200 cm3 (see note 7 below)
- Access to hydrochloric acid, 1 M
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. Take care with hot apparatus and solutions.
- Hydrochloric acid, HCl(aq) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043.
- Limewater, Ca(OH)2(aq), (treat as IRRITANT) – see CLEAPSS Hazcard HC018 and CLEAPSS Recipe Book RB020.
- Calcium sulfate, hydrated, CaSO4.2H2O(s) – see CLEAPSS Hazcard HC019B.
- Sodium chloride, NaCl(s) – see CLEAPSS Hazcard HC047b.
- Although it is tempting to use genuine seawater if available, this experiment is usually more successful if the seawater is generated artificially. Genuine seawater will not always yield a full range of solids in sufficient quantities to be detected. Artificial seawater can be generated by adopting the following procedure:
- Bubble carbon dioxide through a mixture of 250 cm3 limewater plus 750 cm3 deionised water for about 20 minutes or until the cloudy precipitate disappears completely.
- Add as much solid hydrated calcium sulfate as will dissolve.
- Add about 15 g of sodium chloride.
- Stir until all the solid has dissolved, leave to settle and then decant or filter the liquid if necessary.
Procedure
- Place 200 cm3 of seawater in a 250 cm3 beaker.
- Heat and boil the seawater.
- Stop heating when about 60–70 cm3 of liquid remains. Solid will be precipitated during this evaporation process.
- Allow to cool and for any solids to settle.
- Pour the clear liquid into the 100 cm3 beaker, leaving the solids behind.
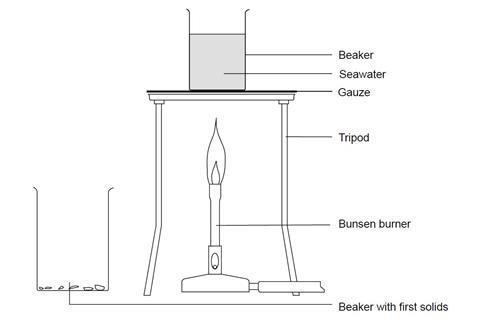
- Add a few drops of hydrochloric acid to the solids left behind and observe what happens.
- Put the 100 cm3 beaker on the tripod and gauze and heat the liquid until another solid appears. This will occur when about 30–40 cm3 of liquid remains.
- Carefully filter the liquid into the conical flask.
- Wash out the 100 cm3 beaker and pour the filtrate into an evaporating basin (or beaker if the volume is too high)
- Boil the liquid yet again until there is almost none left. When a small crust of solid starts to form at the edge of the basin, turn off the Bunsen burner. Then, either allow the remaining water to evaporate at room temperature or place the basin on a beaker half full of water (a steam bath). Heat the beaker with a Bunsen and allow the remaining water in the basin to evaporate gently. SAFETY NOTE: there is a risk of tiny crystals of hot solid spitting out. Eye protection is essential at this stage.
- Let it cool and note what is observed.
Teaching notes
Encourage the students to write down what they observe at each stage.
Teachers may wish to mark 250 cm3 beakers at the 60 cm3 level if there are no gradations already present.
The artificial seawater contains calcium hydrogen carbonate owing to the reaction of limewater with excess carbon dioxide:
Ca(OH)2(aq) + 2CO2(g) → Ca(HCO3)2(aq)
When this solution is boiled it soon precipitates calcium carbonate:
Ca(HCO3)2(aq) → CaCO3(s) + CO2(g) + H2O(l)
This is the identity of the predominant solid first left behind when the liquid is boiled. However, some calcium sulfate will also be present.
When hydrochloric acid is added to this solid, students should observe effervescence (fizzing), since the calcium carbonate is producing carbon dioxide gas:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
The solid which continuously crystallises out on further evaporation is sparingly soluble calcium sulfate, which is the predominant solid filtered off when 30 cm3 of sea water remains.
The more soluble sodium chloride precipitates out during the final stages of evaporation.
Student questions
- What evidence is there that seawater is a mixture of salts?
- What gas is likely to have been evolved when hydrochloric acid is added to the solids first collected?
- What does this tell you about the identity of these solids?
- Do some research on the internet to find information about the solubilities of sodium chloride and calcium sulfate – two common compounds present in seawater. Use this information to predict the possible identity of the very last solid left at the end of your experiment.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet