Try this class practical using paper chromatography to separate and investigate the pigments in a leaf
Most leaves are green due to chlorophyll. This substance is important in photosynthesis (the process by which plants make their food). In this experiment, students investigate the different pigments present in a leaf, from chlorophyll to carotenes, using paper chromatography.
The experiment takes about 30 minutes and can be carried out in groups of two or three students.
Equipment
Apparatus
- Eye protection
- Pestle and mortar
- Chromatography paper
- Beaker, 100 cm3
- Small capillary tube (see note 1)
- Pencil
- Ruler
- Sellotape
- Cut-up leaves, or leaves and scissors (see note 2)
Chemicals
- Propanone (HIGHLY FLAMMABLE, IRRITANT), supplied in a small bottle fitted with a teat pipette (see note 3)
- Sand
Equipment notes
- The capillary tubing can be ‘home-made’ from lengths of ordinary glass tubing (diameter: 3–4 mm) using a Bunsen burner fitted with a flame-spreading (‘fish-tail’) jet.
- A variety of leaves can be used. Best results are obtained from trees or bushes with dark green leaves, eg holly.
- Preferably use teat pipettes that do not allow squirting, eg those fitted to dropper bottles of universal indicator.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Propanone, CH3 COCH3 (l), (HIGHLY FLAMMABLE, IRRITANT) – see CLEAPSS Hazcard HC085A. The vapour of propanone is HIGHLY FLAMMABLE. Do not have any source of ignition nearby.
Procedure
- Finely cut up some leaves and fill a mortar to about 2 cm depth.
- Add a pinch of sand and about six drops of propanone from the teat pipette.
- Grind the mixture with a pestle for at least three minutes.
- On a strip of chromatography paper, draw a pencil line 3 cm from the bottom.
- Use a fine glass tube to put liquid from the leaf extract onto the centre of the line. Keep the spot as small as possible.
- Allow the spot to dry, then add another spot on top. Add five more drops of solution, letting each one dry before putting on the next. The idea is to build up a very concentrated small spot on the paper.
- Attach the paper to the pencil using sellotape so that when placed in the beaker, the paper is just clear of its base.
- Place no more than about 10 cm3 of propanone in the beaker and hang the paper so it dips in the propanone. Ensure the propanone level is below the spot.
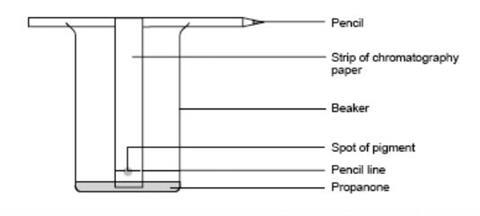
- Avoid moving the beaker in any way once the chromatography has started.
- Leave the experiment until the propanone has soaked near to the top, and then remove the paper from the beaker.
- Mark how high the propanone gets on the paper with a pencil and let the chromatogram dry.
Teaching notes
This experiment works very well providing care is taken over preparing the spot on the chromatography paper. It should be as small and as concentrated as possible. Encourage students to be patient and to wait until each application is dry before adding the next.
At least three spots should be obtained, and one of these should be yellow due to carotenes.
The extent to which any particular component moves up the paper is dependent not only on its solubility in propanone but also on its attraction for the cellulose in the chromatography paper. The yellow carotene spot (with a higher RF value) tends to move up the paper the furthest.
More resources
Add context and inspire your learners with our short career videos showing how chemistry is making a difference.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet