Use this demonstration to produce liquid chlorine and compare it with bromine and iodine in their condensed state
In this experiment, students observe as chlorine gas is passed over a ‘cold finger’ condenser and liquefied using a dry ice/ethanol mixture. Yellow drops of liquid chlorine may then be collected for comparison with other Group 17 elements, specifically bromine and iodine.
The demonstration shows that chlorine gas is relatively easily liquefied (boiling point –35 °C) by cooling alone. It can be used as part of a study of trends in the physical properties of the halogens, and can also be extended to include changes of state for bromine and iodine.
It must be done in a fume cupboard.
The demonstration can be done in five minutes once the chlorine generator is set up and connected to the ‘cold finger’. If the freezing of bromine and the melting and vaporisation of iodine are included allow 15 minutes in total.
Equipment
Apparatus
- Eye protection
- Access to a fume cupboard
- Protective gloves for handling pellets of dry ice (–78 °C) (see note 2 below)
- Chlorine generator (see note 10)
- ‘Cold finger’ condenser (see note 11)
- Bosses, clamps and stands
Optional
- Boiling tube with cotton wool plug
- Test tube holder
- Beaker, 100 cm3
- Bunsen burner
Chemicals
- Sodium chlorate(I) solution, 14% (w/v) available chlorine (CORROSIVE), about 100 cm3 (also known as sodium hypochlorite)
- Hydrochloric acid, 5 M (CORROSIVE), about 50 cm3
- Ethanol (HIGHLY FLAMMABLE) or industrial denatured alcohol (IDA) (HIGHLY FLAMMABLE, HARMFUL), about 20 cm3
- A few small pellets of dry ice (solid carbon dioxide) (can cause serious frostbite if handled without tongs or suitable gloves)
Optional
- Crushed ice, about 100 cm3
- Sodium chloride (crushed rock salt will do), about 100 g
- Bromine liquid (VERY TOXIC, CORROSIVE, DANGEROUS FOR THE ENVIRONMENT) – use a sealed ampoule
- Iodine (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), a few crystals
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout and use protective gloves. Tongs or insulating (not rubber) gloves should be used for handling pellets of dry ice.
- Sodium chlorate(I) solution (also known as Sodium hypochlorite), NaClO(aq), (CORROSIVE at this concentration) – see CLEAPSS Hazcard HC089.
- Hydrochloric acid, HCl(aq), (CORROSIVE at this concentration) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043.
- Chlorine, Cl2(g) and (l), (CORROSIVE, TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC022a and CLEAPSS Recipe Book RB024.
- Ethanol, C2H5OH(l), (HIGHLY FLAMMABLE) or industrial denatured alcohol (IDA) (HIGHLY FLAMMABLE, HARMFUL) – see CLEAPSS Hazcard HC040A.
- Solid carbon dioxide – see CLEAPSS Hazcard HC020a. Dry ice (solid carbon dioxide) can often be obtained from a local university, hospital or industry. Larger chunks can be broken up by enclosing them in a cloth, such as a tea towel, and hitting them with a mallet. The fragments can be stored for several hours in a box made of expanded polystyrene, or in a wide-mouth vacuum flask which was unstoppered (unless it is a vented flask specifically designed for cryogenic work). A more powdery form of dry ice can be made using carbon dioxide from a cylinder and a suitable dry ice making attachment. Tongs or insulating (not rubber) gloves should be used for handling dry ice.
- Bromine, Br2(l), (VERY TOXIC, CORROSIVE, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC015a.
- Iodine, I2(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC054.
- For chlorine generation, see these standard techniques for generating, collecting and testing gases.
- The ‘cold finger’ condenser apparatus should consist of a 1 dm3 Buchner flask fitted with a two-holed rubber bung. One hole in the bung should be big enough to take a test tube and the other hole fitted with a short length of glass delivery tubing – see diagram.
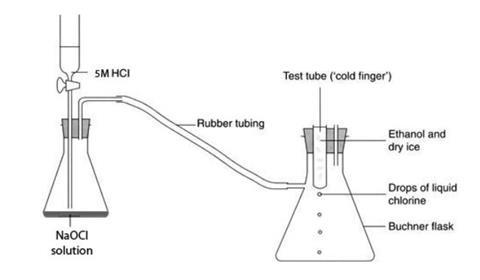
Procedure
- Set up the chlorine generator in a fume cupboard. Make sure it is securely clamped.
- Connect the cold finger apparatus to the generator, using a short length of rubber tubing, and clamp it securely.
- Fill the ‘cold finger’ test tube about two-thirds full of dry ice chips and slowly add a little ethanol. The mixture will bubble vigorously at first as the solid carbon dioxide sublimes. When the bubbling has settled down, add more ethanol until the test tube is almost full. (In CLEAPSS instructions for making freezing mixtures, the dry ice is added to the solvent – with the quantities involved here it is unlikely to matter which way round you add them.)
- Generate a gentle stream of chlorine by dripping the hydrochloric acid slowly on to the sodium chlorate(I). The greenish-yellow gas will gradually fill both flasks.
- After about a minute, yellow drops of liquid chlorine begin to condense on the ‘cold finger’ and drop onto the bottom of the flask. At first these drops will vaporise but after a few minutes they will begin to collect as the base of the flask cools down. Continue passing chlorine gas through the apparatus until sufficient liquid chlorine has collected for the class to see. It is helpful to pre-cool the base of the flask with some dry ice or ice/salt mixture. After stopping the flow of chlorine gas, the flask containing the liquid chlorine can be disconnected from the gas generator but should not be brought out of the fume cupboard.
Teaching notes
Liquid chlorine is transported around the country in bulk in rail or road tankers. Here liquid chlorine can be compared with bromine and iodine as part of a study of the trends in physical properties of the halogens.
This demonstration can be extended to include the freezing of liquid bromine (freezing point –7 °C) and the melting and vaporisation of iodine.
Bromine in a sealed ampoule can be solidified by cooling it in a mixture of equal masses of crushed ice and salt (sodium chloride).
A few crystals of iodine gently heated in a boiling tube (containing a cotton wool plug at the mouth of the tube to prevent the escape of iodine vapour) will melt (melting point 114 °C) and then form a deep purple vapour. On cooling, iodine crystals form on the walls of the tube.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet