Investigate the effect of surface area or concentration on rate of reaction using oxalic acid in rhubarb to reduce and colourise potassium manganate(VII) solution
In this experiment, students use rhubarb sticks, which contain oxalic acid, to reduce and decolourise potassium manganate(VII) solution. The practical includes two parts, which can be used to show how the rate of reaction is affected by surface area and concentration respectively.
The experiment is probably most suited to younger students or groups of students who do not need to be given the details of the reaction itself. It is difficult to relate the rate back to the equation. This is because the results can be complex, due to the competing reactions taking place. (See the teaching notes below.) On a simple level the experiment works well, and makes an unusual alternative to hydrochloric acid and marble chips.
All the practical work can easily be completed in a one-hour session, and graphs could be plotted of the results. Alternatively, the practical work and follow-up can be split over two separate sessions. You could also discuss with students in what ways this experiment is not a fair test.
Warn students not to consume anything in the lab – they may be tempted to taste the rhubarb (although it doesn’t taste very good unsweetened).
If you use rhubarb from home or someone’s garden, ensure that the leaves are removed before it is given to students. Rhubarb leaves contain far more oxalic acid than the stalk, and are HARMFUL.
Equipment
Apparatus
For investigating the effect of surface area
- Eye protection
- Beakers, 100 cm3, x2 or more
- Measuring cylinder, 50 cm3
- Timer
- White tile or piece of paper
- Access to knives, x4–6 per class (ordinary table knives are probably most appropriate)
Additional requirements for investigating the effect of concentration
- Access to filter funnels and filter paper or tea strainers
- Beaker, 250 cm3
- Bunsen burner
- Heat resistant mat
- Tripod
- Gauze
Chemicals
- Rhubarb stalks (frozen rhubarb also works if the pieces are long enough) (see note 5 below)
- For the potassium manganate(VII) solution (see note 6):
- Potassium manganate(VII) (OXIDISING, HARMFUL, DANGEROUS FOR THE ENVIRONMENT), less than 1g
- Dilute sulfuric acid, 1 M, (IRRITANT), volume required depends on the number of student groups
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Potassium manganate(VII), KMnO4(s), (OXIDISING, HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC081.
- Dilute sulfuric acid, H2SO4(aq), (IRRITANT) – see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book RB098.
- Oxalic acid, in the rhubarb (HARMFUL) – see CLEAPSS Hazcard HC036A. As the oxalic acid content of the rhubarb will vary, it is well worth checking that the potassium manganate(VII) solution will give appropriate results with the rhubarb you are using.
- To make the potassium manganate(VII) solution: put 4 or 5 crystals (or sufficient powder to just cover the tip of a spatula) into a 2 dm3 beaker (or large jug) with about 500 cm3 of 1 M sulfuric acid (IRRITANT). Stir until the crystals dissolve. Add a further 500 cm3 of 1 M sulfuric acid. Stir to mix. The solution should be labelled as IRRITANT. The solution should be a light purple colour - if necessary, dilute further with a little more water. The exact concentration is not critical. Each group of students will need approximately 300 cm3 of the solution in total (for both experiments). Adjust the volumes given above accordingly.
Procedure
Investigating the effect of surface area
- Cut three 5 cm lengths of rhubarb. Leave one piece as it is, cut one piece in half lengthways, and cut the third piece into four evenly-sized pieces.
- Measure 30 cm3 of acidified potassium manganate(VII) solution into a beaker. Pour the same quantity of water into another beaker.
- Place the beakers on a white tile. Put the whole 5 cm long piece of rhubarb into the potassium manganate(VII) and start the timer. Stir the solution containing the rhubarb until the purple colour disappears. If you are not sure, briefly remove the rhubarb and compare the colour of the solution to the beaker of water. When they look the same, stop the timer.
- Rinse out and dry the reaction beaker.
- Repeat the experiment using the piece of rhubarb cut into two (use both halves). Rinse and dry the beaker.
- Repeat the experiment again, this time using the piece of rhubarb cut into four.
Investigating the effect of concentration
- Cut the stick of rhubarb (width ways this time) into thin slices (about 0.5 cm) and put them into the 250 cm3 beaker. Cover the rhubarb with distilled water and heat gently.
- Bring the rhubarb to the boil and continue heating gently until the rhubarb falls to pieces. This will take about five minutes. Turn off the Bunsen and leave the rhubarb mixture to cool.
- When cool enough to handle, filter or strain the mixture and KEEP the filtrate (liquid).
- Measure 30 cm3 of acidified potassium manganate(VII) solution into one of the 100 cm3 beakers and the same amount of water into the other.
- Add one drop of the rhubarb filtrate to the potassium manganate(VII) solution and start the timer. Stop the timer when the colour disappears and is the same as the plain water.
- Repeat the experiment for two, three, four, five, and six drops of the rhubarb extract.
Teaching notes
Rhubarb contains oxalic acid (ethanedioic acid) which has the formula C2H2O4.
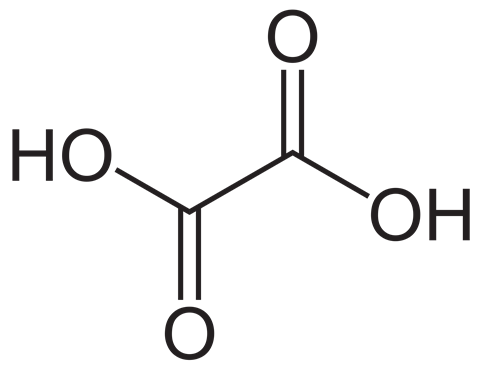
Oxalic acid reacts with potassium manganate(VII) in acidic solutions and is oxidised to carbon dioxide and water:
2MnO4– + 5C2H2O4 + 6H3O+ → 2Mn2+ + 10CO2 + 14H2O
The potassium manganate(VII) decolourises which provides a convenient and easy-to-measure end-point to the reaction. Aqueous solutions of Mn2+ are actually pale pink, but at these concentrations will appear almost colourless.
Students should be able to observe that as the surface area of the rhubarb increases, so does the rate of the reaction. Likewise for increasing concentration of rhubarb juice. The concentration has been varied by putting in more drops of the rhubarb extract, so the total volume has been increased. You may like to discuss the implications of this with the students.
It is worth noting that the reaction is autocatalysed (catalysed by a product of the reaction) by the Mn2+ ions. This could lead to some confusing patterns for students if the results are analysed too closely, and an attempt is made to link the results to the equation.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















2 readers' comments