Use this demonstration to show how pure water can be recovered from copper sulfate solution using a condenser
When copper sulfate solution is boiled, pure water vapour is produced. In this experiment, students observe how this may be captured using a water-cooled condenser, producing liquid water with a boiling point of 100°C.
This demonstration should take about 15 minutes (not including assembling the apparatus), and follows on from another simple experiment which students can try for themselves, using simple distillation to recover water from copper(II) sulfate solution.
Equipment
Apparatus
- Distillation flask, at least 100 cm3 capacity
- Water-cooled (Liebig) condenser and connection tubing to tap and sink
- Corks or bungs to assemble apparatus (or use Quickfit apparatus)
- Thermometer, –10 °C to +110 °C
- Stand, boss and clamp, x2
- Bunsen burner
- Tripod and gauze
- Heat resistant mat
- Beaker, 100 cm3
- Anti-bumping granules (or pumice stone, or pieces of broken porcelain)
Chemicals
- Copper(II) sulfate(VI) solution, 1 M (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), 30 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Copper(II) sulfate(VI) solution, CuSO4(aq), (HARMFUL at this concentration) – see CLEAPSS Hazcard HC027c and CLEAPSS Recipe Book RB031. Copper(II) sulfate(VI) is DANGEROUS FOR THE ENVIRONMENT – recycle the copper sulfate after the demonstration by mixing it with the water that has collected in the beaker.
Procedure
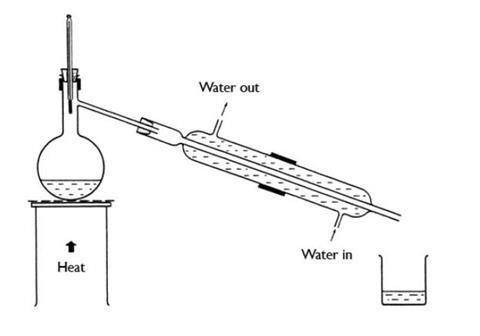
- Set up the apparatus as shown in the diagram, with about 30 cm3 copper(II) sulfate(VI) solution (and a few anti-bumping granules) in the flask.
- Turn on the cooling water. Only a slow flow through the apparatus is needed.
- Heat the copper sulfate solution until it boils, then adjust the flame to keep it boiling gently.
- Read the thermometer as water begins to condense on it and then as the vapour moves down into the condenser.
- Use a beaker to collect the water that runs out of the condenser.
Teaching notes
As in the student experiment based on distilling water from copper sulfate solution, an important point is that the blue solution boils to give the colourless solvent (water). In this experiment, the boiling point of the solvent can be measured and should be steady and close to 100 °C (depending on the accuracy of the thermometer, and the pressure). This is by far the best test for pure water. The use of cobalt(II) chloride paper or anhydrous copper(II) sulfate only indicates the presence of water.
Evaporation occurs at any temperature. Boiling occurs when the vapour pressure of the liquid equals the pressure of the atmosphere. At this temperature, small bubbles (which contain water vapour, not air) are formed in the liquid and rise to the surface. Anti-bumping granules help these bubbles to form. If the granules are not there, the liquid may ‘bump’, ie boil violently.
Point out to the students the mode of action of the water condenser. If water enters at the bottom and comes out at the top, only a slow flow through the condenser is needed.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet