In this class practical, students test the conductivity of covalent and ionic substances in solid and molten states
This experiment enables students to distinguish between electrolytes and non-electrolytes, and to verify that covalent substances never conduct electricity even when liquefied, whereas ionic compounds conduct when molten.
The practical works well as a class experiment, with students working in groups of two to three. There will not be time to investigate all the substances, so each group could be assigned three or four of these, and the results pooled at the end.
Equipment
Apparatus
- Eye protection
- Carbon (graphite) electrodes, fitted in a holder (see note 1 below)
- Bunsen burner
- Tripod
- Pipeclay triangle
- Heat resistant mat
- Clamp and stand
- Small pieces of emery paper
- Connecting leads and crocodile clips
- DC power pack, 6 V
- Light bulb in holder, 6 V (see note 2 below)
Apparatus notes
- The carbon electrodes need to be fixed in some sort of support – such as a polythene holder or large rubber bung – so that there is no possibility of the electrodes being allowed to short-circuit. The electrodes need to be fixed in such a way as to fit inside the crucible supplied.
- A light bulb has more visual impact, but an ammeter can be used instead.
Chemicals
- Small pieces of lead (TOXIC), copper and perhaps other metals
- Crucibles containing samples of:
- Phenylsalicylate (salol) (IRRITANT, DANGEROUS FOR THE ENVIRONMENT)
- Polythene
- Wax
- Sugar
- Zinc chloride (CORROSIVE, DANGEROUS FOR THE ENVIRONMENT)
- Potassium iodide
- Sulfur (optional)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Lead, Pb(s), (TOXIC) – see CLEAPSS Hazcard HC056.
- Copper, Cu(s) – see CLEAPSS Hazcard HC026.
- Phenylsalicylate (salol), C6 H4 (OH)COOC6 H5 (s), (IRRITANT, DANGROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC052.
- Wax – see CLEAPSS Hazcard HC045b.
- Sugar (sucrose), C12 H22 O11 (s) – see CLEAPSS Hazcard HC040c.
- Zinc chloride, ZnCl2 (s) (CORROSIVE, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC108a.
- Potassium iodide, KI(s) - see CLEAPSS Hazcard HC047b.
- Sulfur, S8 (s) – see CLEAPSS Hazcard HC096A. Sulfur is a non-metallic element and is a good substance to have included in the list. But there is a strong likelihood of it catching fire, with sulfur dioxide, SO2 (g), (TOXIC), given off. Sulfur fires are hard to extinguish. If it happens, cover the vessel with a damp cloth and leave in place until cool. If there is time, sulfur can be done as a teacher demonstration. Heat a small sample of ‘flowers of sulfur’ very, very slowly. Sulfur is a very poor conductor of heat, and localised heating is likely to cause it to start burning! You must use a fume cupboard.
Procedure
Part 1
- Set up the circuit as shown in the diagram, at this stage do not include the crucible or bunsen burner flame (these are for later).
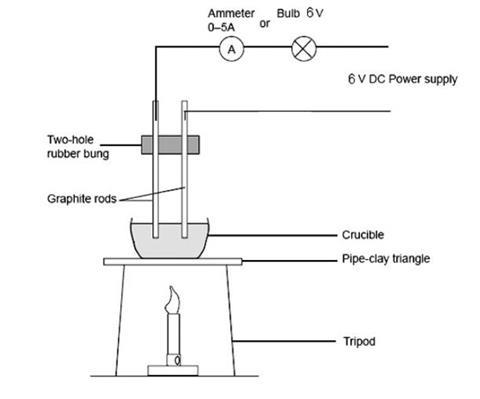
- Select one of the metals, and by holding the electrodes in contact with it, find out whether or not it conducts electricity then switch the current off.
- Note down the results using the student sheet available with this resource (see download links below) and repeat this experiment with each metal available.
- Select one of the solids contained in a crucible. Lower the electrodes so that they are well immersed in the solid, and then clamp the electrodes in position.
- Switch on the current and find out whether the solid conducts electricity or not, then switch the current off again.
- Set the crucible over a Bunsen burner on a pipeclay triangle and tripod, and clamp the electrodes in position over the crucible. Gently heat the sample until it just melts, and then turn off the Bunsen flame. If necessary lower the electrodes into the molten substance, before clamping them again.
- Switch on the current again. Does the molten substance conduct electricity now? Switch the current off again.
- Write up all your observations.
- Raise the electrodes from the crucible, and allow them to cool.
- Clean the electrodes with emery paper.
Part 2
Repeat steps 4 to 10 with some or all of the other solids.
Part 3
Pool your results with other groups so that your table is complete.
Teaching notes
The covalent solids only need to be heated for a short time for melting to take place. Under no circumstances should heating be prolonged, otherwise the substances may decompose and/or burn. The students should be warned about what to do if this happens eg cover with a damp cloth. The experiments should be done in a well-ventilated laboratory.
It may be helpful to reserve a crucible for each of the powdered compounds, while having one or two others that can be heated. Once a solid has been liquefied and allowed to cool, the solidified lump is often hard to break up or powder in the crucible.
Zinc chloride melts at about 285 °C, so heating needs to be fairly prolonged in comparison with the covalent solids. It will, however, produce chlorine (TOXIC) so heating should stop as soon as conductivity is detected. Potassium iodide melts at about 675 °C, so very strong and prolonged heating is needed here.
Student questions
- What do you conclude about the electrical conductivity of metals?
- Do all of the solid compounds conduct electricity?
- Do any of the molten compounds conduct electricity. If so, which ones?
- Why do some substances conduct only when they have been liquefied?
- Can you now classify all the compounds as being either ionic or covalent?
Answers
- All the metals conduct electricity well. You should explain this conductivity in terms of the ‘free’ electrons within a metallic structure.
- No, none of them.
- Yes, zinc chloride and potassium iodide.
- Some substances are ionic, but electrical conduction is only possible when the ions are free and mobile. This happens once the solid has been melted.
- Phenylsalicylate, polythene, wax and sugar are covalent. Zinc chloride and potassium iodide are ionic.
Downloads
Which substances conduct electricity? worksheet
Editable handout | Word, Size 55.06 kbWhich substances conduct electricity? worksheet
Handout | PDF, Size 84.32 kb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet