- Home
- I am a …
- Resources
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- Literacy in science teaching
- More …
- Climate change and sustainability
- Alchemy
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Collections
- Education in Chemistry
- Teach Chemistry
- Events
- Teacher PD
- Enrichment
- Our work
- More navigation items
Acids and bases
Classroom resources from our Acids and Bases professional development course for teachers
This collection is most valuable to those who have attended this course and wish to put into practice with their students some of the ideas and activities presented as part of that event. Please note that this list is not exhaustive; not all trainer activities have a corresponding classroom resource. In some circumstances there is variation between the training resource and classroom resource.
Universal indicator ‘rainbow’
Try this demonstration to create a rainbow effect using universal indicator, hydrochloric acid and sodium hydroxide. Includes kit list and safety instructions.
Neutralisation circles
Support students to explore neutralisation circles in this experiment that can be performed with common chemistry classroom equipment. Kit list and safety instructions included.
Testing the pH of oxides
Use this class practical to investigate the pH of different metal and non-metal oxides using a universal indicator. Includes kit list and safety instructions.
Equilibria involving carbon dioxide in aqueous solution
Use this demonstration or class practical to illustrate changes to equilibria in carbonated soda water. Includes kit list and safety instructions.
A thermometric titration
Use this class practical to practise locating end-points in titration by measuring temperature during the reaction. Includes kit list and safety instructions.
Chemical misconceptions II: Acid strength
Learn more about acid solutions, and distinguishing between concentration and strength.
Universal indicators
This activity develops understanding of universal indicators and single indicators. The students build up their understanding by mixing two indicators. They also develop an awareness that the observed colour may be due to a mixture of colours.
A chromate–dichromate equilibrium
Try this class practical to investigate an equilibrium between chromate(VI), dichromate(VI) and hydrogen ions. Includes kit list and safety instructions.
A microscale acid–base titration
Use microscale titration to complete an acid–base neutralisation with sodium hydroxide in this class practical. Includes kit list and safety instructions.
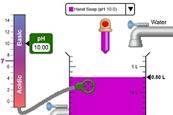
pH scale basics simulation
Explore the basics of pH using this simulation. Add a variety of common solutions, modify the concentration and see the effects on pH.
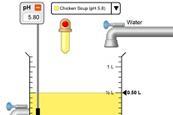
pH scale advanced simulation
Use this advanced pH simulator to view, modify, and visualise solutions with different ion ratios. Explore macroscopic, microscopic, and custom views.
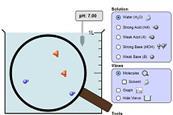
Acid-base solutions simulation
Explore the basics of pH with this simulation. Add a variety of common solutions and modify the concentrations to see the effect on pH.