Determining a compound’s structure can be a puzzle, but these logical steps help students piece it all together
Ask most chemistry students coming to the end of their school education to describe ethanol, and they will be able to describe its familiar CH3CH2OH structure. But how do we know this is correct?
An ethanol molecule has dimensions of approx 4.4 × 10-10 m, or 4.4 Ångstroms. This is far too small to be studied directly. So, chemists have developed a range of instrumental techniques to help spy on what they cannot see.
The first nuclear magnetic resonance (NMR) spectrum of a fluid sample was of ethanol taken at Stanford University in 1951. Here was direct evidence for the existence of three distinct chemical sites with the intensity ratio 1:2:3.
Since then, structure determination has advanced significantly and is now a widely employed tool in materials, chemical and biomolecular research.
Use the accompanying resource Structure determination, with a student sheet and teacher notes available as MS Word documents and PDFs.
Download this
Analyse and interpret spectra with your 16–18 students using the Structure determination resource from the Education in Chemistry website: rsc.li/33Eucv5
What students need to know
To determine the structure of an unknown molecule based on analytical data, students need to have a good understanding of how:
- mass spectrometry can be used to determine the molecular formula of a compound;
- infrared spectroscopy can be used to identify particular covalent bonds in a compound;
- NMR gives information about how atoms are bonded in a compound.
Interpreting spectra and identifying unknowns by spectral analysis requires students to have a secure understanding of the different functional groups that can be found in an organic molecule.
Common misconceptions
Students often struggle to identify which atoms are in the same and which are in different chemical environments and thus appear as separate peaks in the spectrum. It is not unusual for a student to erroneously suggest that the carbon atoms in both CH3 groups in butanone (Figure 1a) are in the same environment owing to the C atom being bonded to 3 × H and 1 × C atom in both.
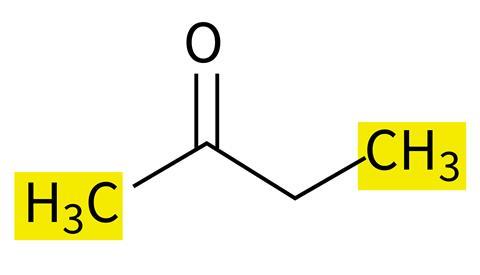
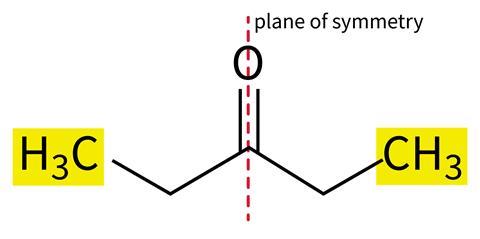
Help students overcome this misconception by encouraging them to look beyond an atom’s immediate connectivity. Use molecular model kits to help visualise this. Emphasise that groups will only be in identical chemical environments in symmetrical molecules, such as pentan-3-one (Figure 1b).
Where students go wrong
Students often incorrectly identify the bond or atom responsible for a peak in the infrared or NMR spectra as a result of misinterpreting information seen on data sheets, such as those provided by exam boards. Before tackling complex problems, ensure students can interpret the data sheet to correctly identify the type of bond or atom referred to.
Students can also fail to ensure that a proposed structure fits all the evidence provided; they may suggest a cyclic alkane as a possible structure when an earlier part of the question indicated that the unknown decolourises bromine water. Careful reading of the exam question and then re-reading once a structure is proposed can help eliminate this.
Ideas
Working out an unknown compound can be like completing a jigsaw puzzle. Looking at all the pieces can seem overwhelming. However by breaking the process down into a series of steps, what seems like an impossible task can become manageable.
Mass spectrometry provides the overall mass of the molecule from which possible molecular formulas can be proposed (the corners). Infrared spectroscopy allows the presence of key functional groups such as carboxylic acids, alcohols, carbonyl compounds and amines to be identified (the edges). 13C and 1H NMR spectroscopy provide information about how these functional groups are connected and the fragmentation pattern from the mass spectrum completes the picture (Figure 2).
Model this process in lessons by introducing one technique at a time and show how each new technique adds to the developing picture.

NMR spectroscopy is a complex spectral technique. Introduce students to 13C NMR first to introduce the idea of unique atom environments and chemical shift without the additional complexities 1H NMR brings. Only teach 1H NMR and the concepts of integration and splitting once students are secure in their understanding of 13C NMR.
To master structure determination, students need plenty of practice. Spectra for a wide range of compounds with varying degrees of difficulty can be downloaded from ChemSpider and used to create problem-solving tasks. Make sure you celebrate with students the sense of satisfaction that arises from the correct identification of an unknown.
Many university chemistry departments offer outreach activities that involve spectroscopy and workshops, so look on university outreach pages or make contact with your local institution to see what they offer.
Assessment
Check students’ understanding by asking them to sketch what they predict the spectra of certain compounds look like before revealing the answer. Increase the complexity as the students develop in competence.
Test students’ ability to suggest chemical structures based on spectral evidence by asking them to identify structures A–H from the Spectroscopy in a Suitcase resource pack.
Challenge students to apply their understanding to more complex problems by tackling these questions from past Olympiad papers.
- Q4 from 2011 requires students to identify the structure of seven compounds with the formula C4H10O based on analytical data (Paper and support materials).
- Q4 from 2005 asks students to assign signals in the 1H NMR spectrum of NanoBalletDancer (Paper and support materials).
Check students understanding by asking them to sketch what they predict the spectra of certain compounds would look like before revealing the actual spectrum. Increase the complexity as the students develop in competence.
Test students ability to suggest chemical structures based on spectral evidence by asking them to identify structures A–H from the Spectroscopy in a Suitcase resource pack (rsc.li/3HTdBm3).
Challenge students to apply their understanding to more complex problems by tackling these questions from past Olympiad papers (rsc.li/3nhISXX).
- Q4 from 2011 requires students to identify the structure of seven compounds with the formula C4H10O based on analytical data.
- Q4 from 2005 asks students to assign signals in the 1H NMR spectrum of NanoBalletDancer.
Take-home points
There is a lot for students to take in when first meeting the topic of structure determination. Build the learning by gradually introducing each new concept one at a time. Show how each new technique adds to the overall picture.
Ensure students have a good understanding of molecular structure and symmetry so they can identify atoms in the same and different chemical environments.
Practice makes perfect! The more students look and work with spectra, the easier the problem-solving process becomes.
Get more resources
- Practise interpreting 1H and 13C NMR, mass spectra, and thin layer chromatograms with these structure determination Starter for ten questions.
- Find out how a scientific associate uses NMR to identify the structure of new and unknown chemical compounds to support the development of new medicines.
- Watch this practical video to link structure determination to testing for organic functional groups.















No comments yet