Watch the video and download the technician notes from the Education in Chemistry website: rsc.li/2KHLKLp
While chemiluminescence can be a great way to show that chemical reactions can release energy as light, rather than heat, photochemical reactions provide opportunities to show the complementary process. The blueprint, or cyanotype, reaction is one of the most famous and lasting demonstrations of this phenomenon. It provides a visually stimulating and memorable activity with a lot of room for creative development.
Kit
The following quantities will generate enough for at least five A4 prints.
- 0.6 g iron(III) chloride-hexahydrate (harmful, irritant, causes serious eye damage)
- 0.6 g citric acid (irritant)
- 0.9 g ammonium carbonate (harmful)
- 1.0 g potassium hexacyanoferrate(III)
- absorbent paper (eg watercolour paper)
- a sponge or paintbrush for applying the mixture to the paper
- hairdryer (electrically tested if brought in from home)
- objects to silhouette or a black and white image printed on acetate film
- a plastic tray (eg a Gratnells tray) for washing in the sink
Preparation
Wear eye protection. Dissolve the hydrated iron chloride and citric acid in 20 cm3 of water. Add the ammonium carbonate in small portions and wait for the frothing to dissipate before continuing to dissolve more.
In front of the class
Add the potassium hexacyanoferrate(III) and dissolve. The mixture is now light sensitive so work in a dim room. Use a sponge or paint brush to apply the mixture to the paper and mop up any excess moisture with lab roll. A hairdryer on a cool setting can finish the drying.
Place the paper in direct sunlight and cover with objects (such as leaves, scissors, or a black and white image on acetate film) to generate a negative image. As the reaction proceeds, the colour of the exposed paper changes from yellow through green to blue. When satisfied by the intensity of the colour (approximately 20 minutes in direct sunlight; a number of hours in diffuse sunlight on a cloudy day), place the paper in a tray under running water and agitate it to thoroughly wash out any unreacted chemicals and leave the negative image as white against blue. The image is now colour-fast and can be hung up to dry.
The paper can be fully prepared ahead of time but must be kept in the dark.
In an earlier Exhibition chemistry, the Magic Beakers demonstration, the last step generates Prussian blue.
Teaching goal
The mixture painted onto the paper, first used by John Herschel in 1842, contains potassium hexacyanoferrate(III) (ferricyanide) and ammonium iron(III) citrate.
The relatively-high energy photons from blue and near-UV light are sufficient to initiate a photochemical redox reaction wherein iron(III) is reduced to iron(II), while the citrate is oxidised to 3-oxopentanedioic acid:
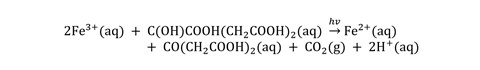
The Prussian blue complex itself precipitates out as a salt containing the mixed oxidation state complex of iron(II) and iron(III), Fe(III)[Fe(II)(CN)6]-. Once the exposure is complete, excess unreacted chemicals are rinsed away, leaving a negative image of white against blue.
Despite the fearsome-sounding name, both the hexacyanoferrate compound and the resulting Prussian blue are low hazard. The same complex stability associated with the ligation of the cyanide ligands to haemoglobin and the resulting toxicity renders them unavailable for release from the compounds encountered in this experiment. Indeed, the ease of production and stability of the blue compound meant it was in use long before the cyanide ligand had been identified, which is how the latter would later acquire its name from the Greek word kyaneos, meaning ‘dark blue’.
Apart from the ease of producing prints from this reaction, the colour fastness of the compound has resulted in the cyanotype process also being used in blueprinting. Photoreduction in the presence of visible light (used, for example, in the silver chloride photographic process) results in iron(III) converting to iron(II). The colour of the Prussian blue compound results from the transfer of electrons between two iron centres of differing oxidation states, so consequently the colour vanishes as iron(III) centres are reduced. However, iron(II) can indeed easily be re-oxidised in air and the blue colour will return, making this dye particularly useful for archival printing.
Safety and disposal
- Wear eye-protection when preparing the cyanotype mixture.
- The mixture and excess starting materials can be washed down a sink with plenty of water. Potassium hexacyanoferrate(III) can release hydrogen cyanide (highly toxic) if brought into contact with strong acids. While this is unlikely to occur during this procedure, care should be taken to ensure that no acid is already present in the drain/sink when disposing of materials.
Downloads
Technician notes
Word, Size 49.02 kbTechnician notes
PDF, Size 0.39 mb

























No comments yet