The reduction, by zinc, of ammonium metavanadate(V) is a staple demonstration. This practical highlights the predictive power of standard electrode potentials. This classic reaction can reveal a beautiful series of greens, blues and violets, but methods suffer from slow reaction times (which means students only get to see the V(II) in a following lesson) or from a lack of clarity caused by small bubbles or additional zinc needed to speed up later reduction steps.
With input from teachers and technicians online I developed a reliable and rapid demonstration of this reaction that gives excellent colour clarity and leaves more time for thinking and further experimentation.
Watch this
A demonstration of this experiment and download the technician notes from the Education in Chemistry website: rsc.li/3XpNbAJ
The reduction, by zinc, of ammonium metavanadate(V) is a staple demonstration. This practical highlights the predictive power of standard electrode potentials. This classic reaction can reveal a beautiful series of greens, blues and violets, but methods suffer from slow reaction times (which means students only get to see the V(II) in a following lesson) or from a lack of clarity caused by small bubbles or additional zinc needed to speed up later reduction steps.
With input from teachers and technicians online (https://twitter.com/declanfleming/status/1600391748114845697), I developed a reliable and rapid demonstration of this reaction that gives excellent colour clarity and leaves more time for thinking and further experimentation.
Kit
- 250 cm3 conical flask or polystyrene cell culture flask
- 0.58 g ammonium metavanadate(V) (safety note: toxic, fatal if inhaled, irritating to skin and eyes)
- 4–6 g zinc powder (safety note: flammable, very toxic to aquatic life with long-lasting effects)
- 100 cm3 2 M hydrochloric acid
- Tea bag (string-tagged cylindrical style)
- Clamp and stand
- 1 L borosilicate beaker
- Hot plate
- Approximately 10 cm3 of 10 vol (3%) hydrogen peroxide in water or 20 cm3 of 0.1 M acidified potassium permanganate(VII)
Preparation
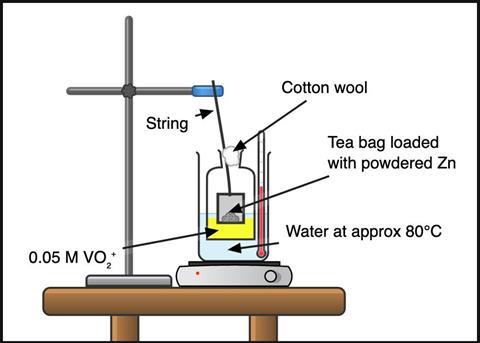
Work in a fume cupboard with the sash down and the extraction off. Wear splash-proof goggles and chemical resistant gloves when handling ammonium vanadate(V) and avoid raising dust. Make up a 0.05 M solution of acidified ammonium vanadate(V) by dissolving 0.58 g in 100 cm3 of 2 M hydrochloric acid. The powder may take a few minutes and some stirring to completely dissolve.
Empty a string-tagged cylindrical-style tea bag and reload it with two to three spatulas of zinc powder (approx 4 g). This is a large excess, but some zinc will be lost to competing reactions (eg with the acid) and some fine powder will escape the bag. Tie the bag with string to make a loop ≈ 30 cm long; this gives plenty of slack to hang the tea bag from a clamp into the reaction flask (see figure 1).
Use a 250 cm³ conical flask to perform this reaction. Or, to better see the colour changes, use a cell culture flask. The flat surface of a cell culture flask provides good visibility, and the oblong shape allows you to visualise the solution through different path lengths to get the best colour saturation.
Place a few centimetres of water into the base of the 1 L beaker and preheat to around 80°C. Boil a kettle of water while you wait.
Safety and disposal notes
- CLEAPSS members should consult HazCard 9B and RB008. Ammonium metavanadate(V) is toxic, and fatal by inhalation. Use this guidance when handling the solid: wear chemical resistant gloves and splash-proof goggles, avoid raising dust and work in a fume cupboard with the extraction off and sash partially lowered.
- For zinc, CLEAPSS members should refer to HC107. Zinc is flammable and very toxic to aquatic life with long-lasting effects. Avoid raising dust and wear eye protection when handling.
- The ammonium metavanadate(V) solution in HCl is an irritant.
- Dispose of the solution of ammonium metavanadate by diluting and pouring down a foul water drain.
- Dispose of any remaining zinc powder by adding to approx 200 cm3 of 1 M acetic acid. Ensure the reaction is complete before pouring down a foul water drain.
In front of the class
Wear eye protection. Load 30–50 cm3 of the acidified vanadate(V) solution into the flask, float it in the water in the beaker and lower the tea bag through the neck above the solution. Top up the beaker with hot water from the kettle so the liquid in the flask is submerged. Adjust the clamp to lower the tea bag so that the base sits near the surface of the acidified vanadate(V) solution.
Below the tea bag, the solution will turn blue, which will appear green as it mixes with the surrounding yellow solution. After a couple of minutes, the solution will be completely blue. It will then change to green and then purple in 10 minutes.
Adding 10 vol hydrogen peroxide, or 0.1 M acidified potassium permanganate(VII) will cause the colour changes to reverse. If you use hydrogen peroxide, you can finish with a flourish with red monoperoxy vanadate, VO(O2)+, as noted in Jennifer Gundersen’s research published in 1986. At this point stop adding oxidants because, in the absence of vanadium(IV), chlorine could be produced.
In front of the class
Wear eye protection. Load 30–50 cm3 of the acidified vanadate(V) solution into the flask, float it in the water in the beaker and lower the tea bag through the neck above the solution. Top up the beaker with hot water from the kettle so the liquid in the flask is submerged. Adjust the clamp to lower the tea bag so that the base sits near the surface of the acidified vanadate(V) solution.
Below the tea bag, the solution will turn blue, which will appear green as it mixes with the surrounding yellow solution. After a couple of minutes, the solution will be completely blue. It will then change to green and then purple in 10 minutes.
Adding 10 vol hydrogen peroxide, or 0.1 M acidified potassium permanganate(VII) will cause the colour changes to reverse. If you use hydrogen peroxide, you can finish with a flourish with red monoperoxy vanadate, VO(O2)+. At this point stop adding oxidants because, in the absence of vanadium(IV), chlorine could be produced.
Teaching goal
This reaction is commonly used to test students’ understanding of the predictability of redox reactions using the electrochemical series. They should be able to predict, with the relevant standard electrode potential data (figure 2), that zinc will couple both with VO2+ and H+ to produce VO2+ and H2, respectively, and that the zinc will continue to reduce the vanadium to V2+.
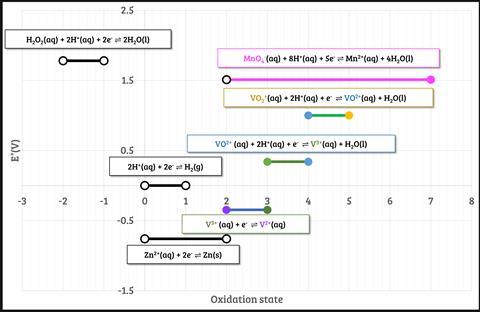
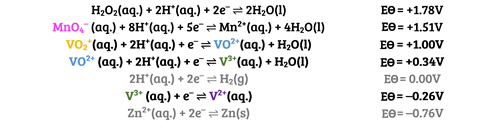
Students can combine individual couples to form ionic equations (Equation 1).
This reaction is commonly used to test students’ understanding of the predictability of redox reactions using the electrochemical series. They should be able to predict, with the relevant standard electrode potential data, that zinc will couple both with VO2+ and H+ to produce VO2+ and H2, respectively, and that the zinc will continue to reduce the vanadium to V2+. Students can combine individual couples to form ionic equations.

There are lots of possibilities of oxidants and reductants. A discussion of alternative strategies can be found in the video.
There are lots of possibilities of oxidants and reductants. Find alternative strategies in the video that accompanies this article.
Top tips
- I work with a more dilute solution – 0.05 M vanadate(V) – rather than the classic 0.1 M because, on a larger scale, the V(III) colour can be too dark to see clearly at this concentration.
- Reactions with granules range from almost non-existent (old school stock will have a surface coating delaying reaction – this can be refreshed by rinsing in 0.1 M copper sulfate solution) to ≈ 1 hour at room temperature.
- Using a tea bag allows you to raise the zinc so that bubbles float up to leave a clear view through the main solution.
- Many methods suggest dissolving the ammonium vanadate(V) in 2 M sodium hydroxide and then making up to a 0.1 M concentration using 1 M sulfuric(VI) acid. That mixture will form V(II) at room temperature in about an hour. You can reduce this time by increasing the temperature and/or switching from granules to powder. When heating the reaction, a brown colour may appear as the V(III) starts to reduce, so add extra acid to reveal the lilac colour – hence my suggestion to make up the solution directly in 2 M hydrochloric acid. It takes longer to dissolve the ammonium vanadate(V) with 2 M HCl than with the 2 M NaOH route but, even with heating, plenty of acid remains to reveal the classic V(II) lilac colour. I suggest the acid swap because although sulfate(VI) ions will be reduced by zinc (Equation 2), chloride ions will not.
Top tips
- I work with a more dilute solution – 0.05 M vanadate(V) – rather than the classic 0.1 M because, on a larger scale, the V(III) colour can be too dark to see clearly at this concentration.
- Reactions with granules range from almost non-existent (old school stock will have a surface coating delaying reaction – this can be refreshed by rinsing in 0.1 M copper sulfate solution) to ≈ 1 hour at room temperature.
- Using a tea bag allows you to raise the zinc so that bubbles float up to leave a clear view through the main solution.
- Many methods suggest dissolving the ammonium vanadate(V) in 2 M sodium hydroxide and then making up to a 0.1 M concentration using 1 M sulfuric(VI) acid. That mixture will form V(II) at room temperature in about an hour. You can reduce this time by increasing the temperature and/or switching from granules to powder. When heating the reaction, a brown colour may appear as the V(III) starts to reduce, so add extra acid to reveal the lilac colour – hence my suggestion to make up the solution directly in 2 M hydrochloric acid. It takes longer to dissolve the ammonium vanadate(V) with 2 M HCl than with the 2 M NaOH route but, even with heating, plenty of acid remains to reveal the classic V(II) lilac colour. I suggest the acid swap because although sulfate(VI) ions will be reduced by zinc, chloride ions will not.

Declan Fleming
Downloads
The vanadate reduction in the bag technician notes
PDF, Size 0.66 mbThe vanadate reduction in the bag technician notes
Word, Size 0.72 mb
























No comments yet