I was looking for details on oscillating reactions and discovered that not only do changes in colour, sound and heat oscillate, but flashes of light from the luminol reaction can also oscillate, creating an eerie lighthouse effect. Like all chemiluminescent reactions, this reaction works best in a darkened room.
Get started
Watch a demonstration of this experiment and download the technician notes from the Education in Chemistry website: rsc.li/3sKk6pl
I was looking for details on oscillating reactions and discovered that not only do changes in colour, sound and heat oscillate, but flashes of light can also oscillate, creating an eerie lighthouse effect. Like all chemiluminescent reactions, this reaction works best in a darkened room.
Kit
- 6 cm3 of 30% (100 vol) hydrogen peroxide and 43 cm3 deionised water (for solution A)
- 0.73 g potassium thiocyanate and 50 cm3 of water (for solution B)
- 0.015 g of copper sulfate pentahydrate salt and 100 cm3 of water (for solution C)
- 0.06 g luminol and 50 cm3 of 0.1 M sodium hydroxide (for solution D)
- 500 cm3 demonstration flask (a beaker, conical or similar)
- 3 x 100 cm3 beakers
- 2 L beaker (for a water bath)
- Kettle
- Magnetic stirrer and stirrer bar
- Thermometer
Preparation
These instructions make a 250 cm3 reaction, but it can be scaled up for a larger audience. Make up the following:
- Solution A: dilute 6 cm3 of 30% (100 vol) hydrogen peroxide to 50 cm3 water to make a 1 M hydrogen peroxide solution.
- Solution B: dissolve 0.73 g of potassium thiocyanate in 50 cm3 of water to make a 0.15 M potassium thiocyanate solution.
- Solution C: dissolve 0.015 g of copper sulfate pentahydrate salt in 100 cm3 of water to make a 6 x 10–4 M copper(II) sulfate solution.
- Solution D: dissolve 0.06 g of luminol in 50 cm3 of 0.1 M sodium hydroxide to make a 7 x 10–3 M alkaline luminol solution.
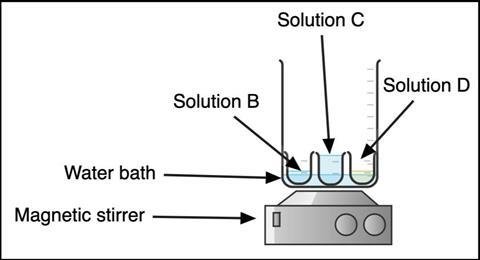
Before the demonstration, boil a kettle of water. Transfer solutions B, C and D into 100 cm3 beakers. Add hot water from the kettle to a larger beaker (I used a 2 L beaker, see Figure 1). Place the beakers of B, C and D into the water bath to heat to 50-60°C, taking care not to float them.
Place solution A into the demonstration flask with the stirrer bar. Darken the room.
Preparation
These instructions make a 250 cm3 reaction, but it can be scaled up for a larger audience. Make up the following:
- Solution A: dilute 6 cm3 of 30% (100 vol) hydrogen peroxide to 50 cm3 water to make a 1 M hydrogen peroxide solution.
- Solution B: dissolve 0.73 g of potassium thiocyanate in 50 cm3 of water to make a 0.15 M potassium thiocyanate solution.
- Solution C: dissolve 0.015 g of copper sulfate pentahydrate salt in 100 cm3 of water to make a 6 x 10–4 M copper(II) sulfate solution.
- Solution D: dissolve 0.06 g of luminol in 50 cm3 of 0.1 M sodium hydroxide to make a 7 x 10–3 M alkaline luminol solution.
Before the demonstration, boil a kettle of water. Transfer solutions B, C and D into 100 cm3 beakers. Add hot water from the kettle to a larger beaker (I used a 2 L beaker). Place the beakers of B, C and D into the water bath to heat to 50-60°C, taking care not to float them.
Place solution A into the demonstration flask with the stirrer bar. Darken the room.
Health, safety and disposal
- Wear eye protection.
- Hydrogen peroxide can cause serious eye damage. CLEAPSS members should see RB045 before diluting 100 vol (30%) hydrogen peroxide.
- Copper(II) sulfate is harmful if swallowed and can cause serious eye damage. Avoid contact with skin.
- Avoid raising dust when measuring out powders.
- Potassium thiocyanate is harmful. Only heat the solution with a water bath and avoid boiling dry.
- Luminol is an irritant (affects the skin and eyes and possibly the respiratory tract).
- Dilute all solutions with plenty of water and dispose in a foul-water drain.
Health and safety and disposal
- Wear eye protection.
- Hydrogen peroxide can cause serious eye damage. CLEAPSS members should see RB045 (bit.ly/46MzwYE) before diluting 100 vol (30%) hydrogen peroxide.
- Copper (II) sulfate is harmful if swallowed and can cause serious eye damage. Avoid contact with skin.
- Avoid raising dust when measuring out powders.
- Potassium thiocyanate is harmful. Only heat the solution with a water bath and avoid boiling dry.
- Luminol is an irritant (affects the skin and eyes and possibly the respiratory tract).
- Dilute all solutions with plenty of water and dispose in a foul-water drain.
In front of the class
When solutions B–D are between 50–60°C, remove them and adjust the temperature of the water bath to 40–50°C.
Ensure the water level is low enough not to float the demonstration flask.
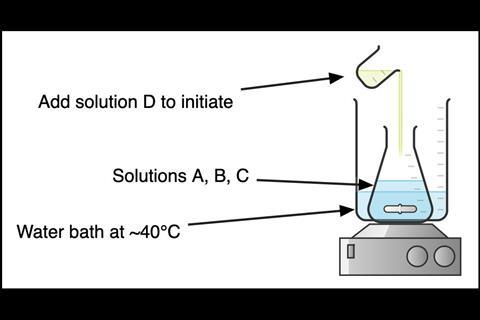
Add solutions B and C to the demonstration flask, then place it into the water bath and stir (see Figure 2). To initiate the reaction, add solution D.
There will be an initial flash of blue light, then a faint afterglow associated with a separate reaction of luminol with superoxide ions in solution. Flashes will repeat. At ~40°C, the reaction lasts 10–15 minutes with steadily increasing periods ranging from approx 30 seconds initially to 70 seconds after 12 minutes (see Figure 3).
In front of the class
When solutions B–D are between 50–60°C, remove them and adjust the temperature of the water bath to 40–50°C. Ensure the water level is low enough not to float the demonstration flask.
Add solutions B and C to the demonstration flask, then place it into the water bath and stir. To initiate the reaction, add solution D.
There will be an initial flash of blue light, then a faint afterglow associated with a separate reaction of luminol with superoxide ions in solution. Flashes will repeat. At ~40°C, the reaction lasts 10–15 minutes with steadily increasing periods ranging from approx 30 seconds initially to 70 seconds after 12 minutes.
Tips
- Use freshly diluted hydrogen peroxide solution.
- Test the respective volumes of the water bath and the anticipated volumes of liquid of B, C and D beforehand. Use 50 cm3 of water in each of the smaller flasks as a stand-in to gauge the float risk before heating the actual solutions. This will help avoid spillage.
- Pre-heat the magnetic stirrer plate by placing the hot water bath of solutions B, C and D on top of it. Note these solutions do not need to be stirred at this point.
- Adjust the scale and temperature of the reaction to suit but avoid temperatures >65°C that quench the oscillator. At higher temperatures, pulses are more frequent and the reaction finishes faster. At room temperature, pulses appear at two-minute intervals.
- With small stirrer beads, you can split the mixture into multiple reactions, so there is less of a delay between flashes.
Teaching goal
This reaction is a great discussion starter, where students will find the predictable intervals of light captivating, like a slow-motion firefly. All oscillating reactions rely on competing autocatalytic steps broadly fitting the following scheme.
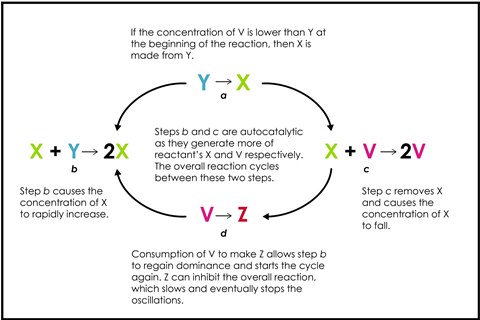
The overall reaction cycles between two central competing reactions that give rise to the oscillations. Over time, the concentration of A falls, so step (b) begins to fail, or the final product D inhibits the reaction, which slows and eventually stops the oscillations.
Briggs–Rauscher and Belousov–Zhabotinskii oscillating systems are more well known, but this reaction follows the Orban oscillating system. The mechanism with luminol has up to 30 different reactions and 26 different variables so we need to simplify it.
Although a dark room is best, typically the audience will see the initial burst of light in a classroom setting. Thereafter the solution turns yellow, which fades moments before the next flash of light. The yellow is attributed to copper(I) complexes and suggests that the copper sulfate has catalysed the decomposition of hydrogen peroxide, but before the copper(I) can be re-oxidised by dissolved oxygen or hydrogen peroxide, it is trapped by strong complexation from thiocyanate ions in the mixture. Copper(II) is needed to catalyse the separate luminol reaction (see Lighting up copper) in its own oxidation by hydrogen peroxide, so as long as the yellow colour is visible, the blue glow will not be.
Meanwhile, hydrogen peroxide and thiocyanate ions undergo a series of reactions to generate OS(O)CN•, which re-oxidises the Cu(I)[SCN]n complexes briefly before the copper(II) is reduced and trapped again.
A range of products are produced including oxygen from the decomposition of the peroxide, sulfate(VI) ions, hydrogen carbonate ions and, crucially, ammonium ions. Therefore, potassium thiocyanate and sodium hydroxide should not be substituted for ammonium compounds that can inhibit the oscillator.
Teaching goal
This reaction is a great discussion starter, where students will find the predictable intervals of light captivating, like a slow-motion firefly. All oscillating reactions rely on competing autocatalytic steps broadly fitting the Brusselator model (bit.ly/3usCu6O). This model comprises four reactions with two central competing reactions that share a reactant and give rise to the oscillations. The overall reaction continually switches between these two central competing reactions.
Although a dark room is best, typically the audience will see the initial burst of light in a classroom setting. Thereafter the solution turns yellow, which fades moments before the next flash of light. The yellow is attributed to copper(I) complexes and suggests that the copper sulfate has catalysed the decomposition of hydrogen peroxide, but before the copper(I) can be re-oxidised by dissolved oxygen or hydrogen peroxide, it is trapped by strong complexation from thiocyanate ions in the mixture. Copper(II) is needed to catalyse the separate luminol reaction (see Lighting up copper, rsc.li/47HEkiq) in its own oxidation by hydrogen peroxide, so as long as the yellow colour is visible, the blue glow will not be.
Meanwhile, hydrogen peroxide and thiocyanate ions undergo a series of reactions to generate OS(O)CN•, which re-oxidises the Cu(I)[SCN]n complexes briefly before the copper(II) is reduced and trapped again.
A range of products are produced including oxygen from the decomposition of the peroxide, sulfate(VI) ions, hydrogen carbonate ions and, crucially, ammonium ions. Therefore, potassium thiocyanate and sodium hydroxide should not be substituted for ammonium compounds that can inhibit the oscillator.
More resources
Looking for more practicals showcasing oscillations, transition metal chemistry and thermodynamics? Check out the following demonstrations:
- The Briggs–Rauscher oscillating system highlights catalysis and the effect of concentration on reaction progress. Suitable for 11–14 and 14–16 learners.
- The Lighting up copper experiment reinforces magnetic properties, variable oxidation states and other transition metal properties. Suitable for 16–18 students.
- The jaw-dropping Luminol fountain provides the opportunity to investigate the properties of ammonia and solubility of gases with 14–16 classes.
- The Let’s get fizzical practical can be used to show your 16–18 students supersaturated solutions and kinetic vs thermodynamic effects.
Downloads
Luminol lighthouse technician notes
Editable handout | Word, Size 0.45 mbLuminol lighthouse technician notes
Handout | PDF, Size 0.15 mb

























No comments yet