- Home
- I am a …
- Resources
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- Literacy in science teaching
- More …
- Climate change and sustainability
- Alchemy
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Collections
- Education in Chemistry
- Teach Chemistry
- Events
- Teacher PD
- Enrichment
- Our work
- More navigation items
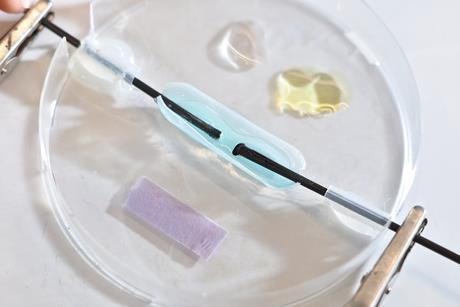
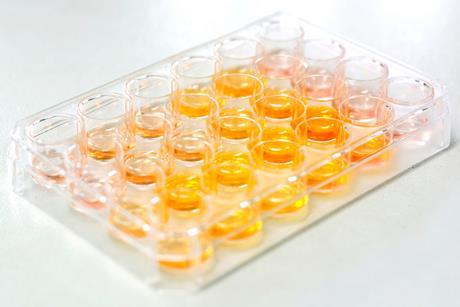
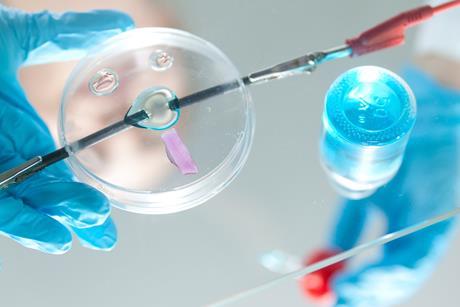
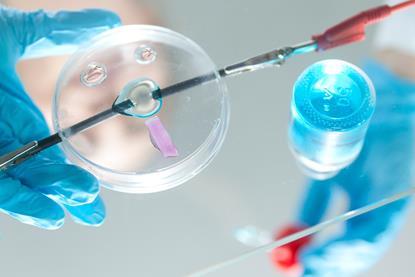
Microscale chemistry
Tips, ideas and practical experiments to help you make the most of microscale chemistry in your classroom
Getting started
The fast guide to microscale practical work
Every chemistry teacher’s must-have guide to this no-fuss method for practical work
Apparatus and techniques for microscale chemistry
Find out what apparatus you need for common microscale experiments, learn about key techniques and discover how to prepare solutions of different elements.
Why microscale?
Maximise learning with microscale
Find out why and how a small-scale approach to practical work reaps big rewards in your classroom
Making practical work more effective
Microscale chemistry and well-ordered teaching sequences reduce cognitive overload
Why I love microscale
Advocating a practical approach to practical work in the classroom
Why microscale?
Maximise learning with microscale
Find out why and how a small-scale approach to practical work reaps big rewards in your classroom
Making practical work more effective
Microscale chemistry and well-ordered teaching sequences reduce cognitive overload
Why I love microscale
Advocating a practical approach to practical work in the classroom
More from Education in Chemistry
Escape the classroom: and try microscale chemistry
Escape room puzzles based on simple microscale reactions for students
More from Education in Chemistry
Escape the classroom: and try microscale chemistry
Escape room puzzles based on simple microscale reactions for students
Microscale chemistry practicals
Electrolysis on a microscale | 14–16 years
Use this microscale version of the electrolysis of copper(II) chloride to safely explore a key practical experiment
Microscale titration | 11–16 years
Use this simple microscale titration experiment with integrated instructions to introduce your learners to titrations
Universal indicator microscale | 11–16 years
Introduce learners aged 11–16 to the concept of pH and address common misconceptions regarding the use of indicators
Microscale diffusion of a gas | 11–16 years
Explore diffusion with this simple and effective microscale experiment
Microscale neutralisation and precipitation reactions | 11–16 years
Hone your learners’ observation skills with two microscale reactions: neutralising citric acid and creating a lead iodide precipitate
Transition elements and complex compounds microscale experiment | 16–18 years
Try this microscale practical investigating the transition elements, complex formation and change in oxidation state. Includes kit list and safety instructions
Practical potions microscale | 11–14 years
Observe chemical changes in this microscale experiment with a spooky twist.
Microscale technicians in trouble! investigation
Some solutions have been mixed up – help the technicians work out which is which
Precipitation reactions of lead nitrate
Compare the colours of various lead compounds to identify which would be good pigments in this microscale practical. Includes kit list and safety instructions.
Some reactions of sulfur dioxide
Observe the reactions of sulfur dioxide with potassium manganate (IV), iodide/iodate mixture and indicator solution. Includes kit list and safety instructions.
The determination of copper in brass
Try this microscale class practical to investigate how much copper there is in brass using nitric acid. Includes kit list and safety instructions.
Microscale reactions of hydrogen sulfide
Observe the reactions of hydrogen sulfide with lead nitrate, silver nitrate and potassium manganate(VII) in this microscale practical. Includes kit list and safety instructions.
Microscale reactions of ammonia
Try this practical to explore the reactions of ammonia with indicator solution, copper(II) sulfate solution and Nessler’s reagent. Includes kit list and safety instructions.
Measuring density
By measuring the relative mass of seawater and tap water, students will be able to discover the density of these liquids. Includes kit list and safety instructions.
The chemistry of thiosulfate ions
Sodium thiosulfate has several interesting reactions with a variety of chemicals. This experiment will let students explore and record these reactions. Includes kit list and safety instructions.
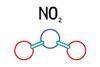
Some reactions of nitrogen dioxide
Using a range of chemicals and solutions, students can create an experiment that will explore some of the reactions of nitrogen dioxide. Includes kit list and safety instructions.
Testing acids and bases on a microscale
Test various substances with indicator solution and look for colour changes in this microscale class practical. Includes kit list and safety instructions.
Mass changes in chemical reactions
Perform two chemical reactions to see whether any mass changes occur in this microscale class practical. Includes kit list and safety instructions.
The oxidation of cyclohexanol by nitric acid
Perform a ring opening oxidation using nitric acid to produce the dicarboxylic acid, 1,6-hexanedioic acid (adipic acid) – and then use the solid crystals that form to determine a melting point. Includes kit list and safety instructions.
Exploring the chemistry of chromium, molybdenum and tungsten
Discover how transition elements differ in aspects of colour, precipitate formation, changes in oxidation state and equilibria. Includes kit list and safety instructions.
Brady’s test for aldehydes and ketones
Identify aldehydes and ketones using Brady’s reagent (2,4-dinitrophenylhydrazine) in this microscale experiment. Includes kit list and safety instructions.
The chemical properties of phenol
Observe and interpret some of the chemical reactions of hydroxybenzene (phenol), by adding five different substances to a Petri dish, and noting down findings. Includes kit list and safety instructions.
Preparing ethyne on a microscale
Generate ethyne gas with calcium carbide and test its properties in this microscale class practical. Includes kit list and safety instructions.
Observing chemical changes
Try this microscale practical to explore the chemical changes in displacement, redox and precipitation reactions. Includes kit list and safety instructions.
Diffusion of gases and relative molecular mass
Try this class practical to explore the diffusion of gases and how relative molecular mass affects rate of diffusion. Includes kit list and safety instructions.
Redox chemistry with dichromate ions
Observe the colour changes that occur with the reduction of dichromate ions by hydrogen peroxide. Includes kit list and safety instructions.
Oxidation states of iron
Compare the two main oxidation states of iron and consider explanations for differences in this microscale practical. Includes kit list and safety instructions.
Microscale reactions of metals with acids
Try this class practical to explore reactivity series with various metals as they react with acids on a microscale. Includes kit list and safety instructions.
Unsaturation test with potassium manganate(VII)
Use a solution of potassium manganate to test for unsaturation in organic compounds in this microscale practical. Includes kit list and safety instructions.
Properties of group 2 elements
Microscale experiment where various anion solutions are added to drops of group 2 element cations. Includes kit list and safety instructions.
Testing for unsaturation with bromine on a microscale
Try this class experiment to prepare elemental bromine and use it to test for unsaturation in organic compounds. Includes kit list and safety instructions.
Oxygen and methylene blue
Reacting hydrogen peroxide, and potassium manganate together will produce detectable oxygen so by using methylene blue solution, and a gas generating apparatus students can test for the presence of oxygen in this practical. Includes kit list and safety instruction.
Synthesis of aspirin on a microscale
Use this class practical to produce aspirin in a microscale esterification reaction using phosphoric acid as a catalyst. Includes kit list and safety instructions.
Energy changes in neutralisation
Study energy changes in two chemical reactions using thermometer strips to measure temperature in this experiment. Includes kit list and safety instructions.
Formation of TCP (2,4,6-trichlorohydroxybenzene)
Delve into preparing TCP by reacting hydroxybenzene (phenol) with chlorine gas, and create this distinctive smelling compound.
Investigating redox reactions on a microscale
Carry out two redox reactions and observe and interpret the results in this microscale class practical. Includes kit list and safety instructions.
The microscale synthesis of indigo dye
Carry out a microscale organic synthesis, the result of which will leave students with indigo dye. Includes kit list and safety instructions.
Finding out how much salt there is in seawater
Use the microscale titration apparatus to titrate silver nitrate solution against sea water
The treatment of oil spills
Tackle the real-life environment problem of oil spills in your classroom, by creating and then treating a micro version of an oil event. Includes kit list and safety instructions.
Some reactions of carbon dioxide
Create carbon dioxide from marble chips and acid, then test for its reaction with barium hydroxide by observing the carbonate precipitate. Includes kit list and safety instructions.
The microscale synthesis of azo dyes
Synthesise an azo dye, and use it to change the colour of cotton, with this class experiment. Includes kit list and safety instructions.
Sulfate and carbonate solubility of Groups 1 and 2
Try this microscale practical to explore the properties of elements in Groups 1 and 2 as they form various precipitates. Includes kit list and safety instructions.
Measuring an equilibrium constant on a microscale
Use your microscale titration apparatus to determine the equilibrium constant for the reaction between silver(I) and iron(II) ions
Exploring the properties of the carvones
Test the smell of each enantiomer of carvone and detect the differences
Measuring the amount of vitamin C in fruit juices
Explore ascorbic acid in fruit juices through titration in this experiment, with specimen results and calculations, stock solutions, and detailed notes included.
Displacement reactions of metals on a microscale
Examine the reactions between various metals and metal salt solutions in this microscale class practical. Includes kit list and safety instructions.
Electrolysis using a microscale Hoffman apparatus
Investigate the electrolysis of sodium sulfate solution using a microscale Hoffman apparatus in this class practical. Includes kit list and safety instructions.
The chemistry of silver
Discover the properties of silver compounds with redox reactions, complex formation and colour/state changes. Includes kit list and safety instructions.
Analysis of aspirin tablets on a microscale
Try this microscale class practical to analyse aspirin tablets and find out how much salicylic acid is present. Includes kit list and safety instructions.
The temperature changes induced by evaporation
Explore the rate of evaporation for a trio of liquids, using just a temperature strip, and our worksheet. Includes kit list and safety instructions.
Properties of stereoisomers
By soaking cotton wool in two limonene enantiomers, and adding a stereoisomer, students can explore the differences between each chemical and discuss how they each might react in different conditions. Includes kit list and safety instructions.
Using a microscale conductivity meter
Explore electrical conductivity with this practical that allows students to test different materials for how well a current will pass through them. Includes kit list and safety instructions.
Microscale extraction of copper
Try this practical to reduce copper(II) oxide to copper using hydrogen, revealing their positions in the reactivity series. Includes kit list and safety instructions.
Electrolysis on a microscale | 14–16 years
Use this microscale version of the electrolysis of copper(II) chloride to safely explore a key practical experiment
Microscale titration | 11–16 years
Use this simple microscale titration experiment with integrated instructions to introduce your learners to titrations
Universal indicator microscale | 11–16 years
Introduce learners aged 11–16 to the concept of pH and address common misconceptions regarding the use of indicators
Microscale diffusion of a gas | 11–16 years
Explore diffusion with this simple and effective microscale experiment
Microscale neutralisation and precipitation reactions | 11–16 years
Hone your learners’ observation skills with two microscale reactions: neutralising citric acid and creating a lead iodide precipitate
Transition elements and complex compounds microscale experiment | 16–18 years
Try this microscale practical investigating the transition elements, complex formation and change in oxidation state. Includes kit list and safety instructions
Practical potions microscale | 11–14 years
Observe chemical changes in this microscale experiment with a spooky twist.
Microscale technicians in trouble! investigation
Some solutions have been mixed up – help the technicians work out which is which
Precipitation reactions of lead nitrate
Compare the colours of various lead compounds to identify which would be good pigments in this microscale practical. Includes kit list and safety instructions.
Some reactions of sulfur dioxide
Observe the reactions of sulfur dioxide with potassium manganate (IV), iodide/iodate mixture and indicator solution. Includes kit list and safety instructions.
The determination of copper in brass
Try this microscale class practical to investigate how much copper there is in brass using nitric acid. Includes kit list and safety instructions.
Microscale reactions of hydrogen sulfide
Observe the reactions of hydrogen sulfide with lead nitrate, silver nitrate and potassium manganate(VII) in this microscale practical. Includes kit list and safety instructions.
Microscale reactions of ammonia
Try this practical to explore the reactions of ammonia with indicator solution, copper(II) sulfate solution and Nessler’s reagent. Includes kit list and safety instructions.
Measuring density
By measuring the relative mass of seawater and tap water, students will be able to discover the density of these liquids. Includes kit list and safety instructions.
The chemistry of thiosulfate ions
Sodium thiosulfate has several interesting reactions with a variety of chemicals. This experiment will let students explore and record these reactions. Includes kit list and safety instructions.
Some reactions of nitrogen dioxide
Using a range of chemicals and solutions, students can create an experiment that will explore some of the reactions of nitrogen dioxide. Includes kit list and safety instructions.
Testing acids and bases on a microscale
Test various substances with indicator solution and look for colour changes in this microscale class practical. Includes kit list and safety instructions.
Mass changes in chemical reactions
Perform two chemical reactions to see whether any mass changes occur in this microscale class practical. Includes kit list and safety instructions.
The oxidation of cyclohexanol by nitric acid
Perform a ring opening oxidation using nitric acid to produce the dicarboxylic acid, 1,6-hexanedioic acid (adipic acid) – and then use the solid crystals that form to determine a melting point. Includes kit list and safety instructions.
Exploring the chemistry of chromium, molybdenum and tungsten
Discover how transition elements differ in aspects of colour, precipitate formation, changes in oxidation state and equilibria. Includes kit list and safety instructions.
Brady’s test for aldehydes and ketones
Identify aldehydes and ketones using Brady’s reagent (2,4-dinitrophenylhydrazine) in this microscale experiment. Includes kit list and safety instructions.
The chemical properties of phenol
Observe and interpret some of the chemical reactions of hydroxybenzene (phenol), by adding five different substances to a Petri dish, and noting down findings. Includes kit list and safety instructions.
Preparing ethyne on a microscale
Generate ethyne gas with calcium carbide and test its properties in this microscale class practical. Includes kit list and safety instructions.
Observing chemical changes
Try this microscale practical to explore the chemical changes in displacement, redox and precipitation reactions. Includes kit list and safety instructions.
Diffusion of gases and relative molecular mass
Try this class practical to explore the diffusion of gases and how relative molecular mass affects rate of diffusion. Includes kit list and safety instructions.
Redox chemistry with dichromate ions
Observe the colour changes that occur with the reduction of dichromate ions by hydrogen peroxide. Includes kit list and safety instructions.
Oxidation states of iron
Compare the two main oxidation states of iron and consider explanations for differences in this microscale practical. Includes kit list and safety instructions.
Microscale reactions of metals with acids
Try this class practical to explore reactivity series with various metals as they react with acids on a microscale. Includes kit list and safety instructions.
Unsaturation test with potassium manganate(VII)
Use a solution of potassium manganate to test for unsaturation in organic compounds in this microscale practical. Includes kit list and safety instructions.
Properties of group 2 elements
Microscale experiment where various anion solutions are added to drops of group 2 element cations. Includes kit list and safety instructions.
Testing for unsaturation with bromine on a microscale
Try this class experiment to prepare elemental bromine and use it to test for unsaturation in organic compounds. Includes kit list and safety instructions.
Oxygen and methylene blue
Reacting hydrogen peroxide, and potassium manganate together will produce detectable oxygen so by using methylene blue solution, and a gas generating apparatus students can test for the presence of oxygen in this practical. Includes kit list and safety instruction.
Synthesis of aspirin on a microscale
Use this class practical to produce aspirin in a microscale esterification reaction using phosphoric acid as a catalyst. Includes kit list and safety instructions.
Energy changes in neutralisation
Study energy changes in two chemical reactions using thermometer strips to measure temperature in this experiment. Includes kit list and safety instructions.
Formation of TCP (2,4,6-trichlorohydroxybenzene)
Delve into preparing TCP by reacting hydroxybenzene (phenol) with chlorine gas, and create this distinctive smelling compound.
Investigating redox reactions on a microscale
Carry out two redox reactions and observe and interpret the results in this microscale class practical. Includes kit list and safety instructions.
The microscale synthesis of indigo dye
Carry out a microscale organic synthesis, the result of which will leave students with indigo dye. Includes kit list and safety instructions.
Finding out how much salt there is in seawater
Use the microscale titration apparatus to titrate silver nitrate solution against sea water
The treatment of oil spills
Tackle the real-life environment problem of oil spills in your classroom, by creating and then treating a micro version of an oil event. Includes kit list and safety instructions.
Some reactions of carbon dioxide
Create carbon dioxide from marble chips and acid, then test for its reaction with barium hydroxide by observing the carbonate precipitate. Includes kit list and safety instructions.
The microscale synthesis of azo dyes
Synthesise an azo dye, and use it to change the colour of cotton, with this class experiment. Includes kit list and safety instructions.
Sulfate and carbonate solubility of Groups 1 and 2
Try this microscale practical to explore the properties of elements in Groups 1 and 2 as they form various precipitates. Includes kit list and safety instructions.
Measuring an equilibrium constant on a microscale
Use your microscale titration apparatus to determine the equilibrium constant for the reaction between silver(I) and iron(II) ions
Exploring the properties of the carvones
Test the smell of each enantiomer of carvone and detect the differences
Measuring the amount of vitamin C in fruit juices
Explore ascorbic acid in fruit juices through titration in this experiment, with specimen results and calculations, stock solutions, and detailed notes included.
Displacement reactions of metals on a microscale
Examine the reactions between various metals and metal salt solutions in this microscale class practical. Includes kit list and safety instructions.
Electrolysis using a microscale Hoffman apparatus
Investigate the electrolysis of sodium sulfate solution using a microscale Hoffman apparatus in this class practical. Includes kit list and safety instructions.
The chemistry of silver
Discover the properties of silver compounds with redox reactions, complex formation and colour/state changes. Includes kit list and safety instructions.
Analysis of aspirin tablets on a microscale
Try this microscale class practical to analyse aspirin tablets and find out how much salicylic acid is present. Includes kit list and safety instructions.
The temperature changes induced by evaporation
Explore the rate of evaporation for a trio of liquids, using just a temperature strip, and our worksheet. Includes kit list and safety instructions.
Properties of stereoisomers
By soaking cotton wool in two limonene enantiomers, and adding a stereoisomer, students can explore the differences between each chemical and discuss how they each might react in different conditions. Includes kit list and safety instructions.
Using a microscale conductivity meter
Explore electrical conductivity with this practical that allows students to test different materials for how well a current will pass through them. Includes kit list and safety instructions.
Microscale extraction of copper
Try this practical to reduce copper(II) oxide to copper using hydrogen, revealing their positions in the reactivity series. Includes kit list and safety instructions.
Practical videos
Video resources featuring microscale experiments, designed to support flipped learning and live practical work
Displacement reactions – practical videos | 14–16 students
Video and resources investigating the displacement reactions between the halogens chlorine, bromine and iodine and their respective halides in microscale
Identifying ions – practical videos | 14–16 students
Video resource showing how to identify ions in various solutions. Flame tests, sodium hydroxide test (microscale) and tests for negative ions: carbonate, sulfate and halide ions
Reactivity of metals | practical videos | 14–16 years
Video with supporting resources featuring three experiments investigating the relative reactivity of metals, including metal displacement reactions in microscale
Electrochemical cells | practical videos | 16–18 students
Video and supporting resources to support electrochemistry practical work, including two microscale experiments, animation and cell diagrams
Exhibition Chemistry demonstrations
Engage and inspire your students with these exciting demonstrations, including videos, practical guidance and teaching notes
The movement of ions: bringing electrolysis to life
Demonstrate the movement of positive and negative ions with a simpler, safer version of this classic demo
Dynamite soap: The combustion of stoichiometric hydrogen–oxygen mixtures
Add this quick demo to the end of a lesson on squeaky pops to show the dramatic impact of mixing chemicals in the correct proportions
About this collection
A number of the practicals and demonstrations featured on this page are based on experiments previously published in Microscale chemistry: experiments in miniature. Royal Society of Chemistry members receive a 35% discount at the RSC bookshop.