How to help your students link conceptual levels
Have you ever been certain your students have understood something fully because they’ve done well in tests and given confident answers to questions, only to find out they’ve misunderstood a fundamental underlying concept? Or have you heard students coming out of an exam saying ‘we didn’t learn about that’, when you know they did; they just failed to make the necessary connection between different ideas?
As teachers, we want to find ways of probing beneath the surface to find out what our students are really thinking and whether they truly understand what we’ve taught them. And we need to help them make connections between ideas that are linked by processes we can’t see, but which experienced chemists can visualise easily.
Making connections
Teachers constantly identify connections between ideas that share similar underlying mechanisms and concepts, but this thinking can be so embedded in our practice we don’t think to explicitly explain it to our students.
For example, when I teach my year 10 students about ions, I know we’ll be talking about displacement reactions, electrolysis and methods for making water potable in the future. During each of these topics, I visualise ions in solution, which helps me to make conceptual links between topics. But my students are unlikely to do this without me prompting. Without help and support, our students will tend to overlook similarities in underlying concepts. They might also view chemistry problems in terms of symbols and equations, rather than visualising the atoms and reactions behind them.
Experienced chemists move fluently between conceptual levels, connecting processes you observe (macroscopic level) with things you can’t see (sub-microscopic level) and using symbols to represent their ideas (symbolic level), summarised by Johnstone’s triangle.
For example, a few years ago, my students had a breakthrough in their understanding of ionic equations, when they finally connected them to something they’d observed. Before this, they’d successfully used an algorithm approach to simplify equations, but they’d been crossing out ‘spectator ions’ without really understanding why they were ‘spectating’.
We carried out some simple precipitation reactions (macroscopic), drew pictures of the ions that were present in solution at key points (sub-micrsoscopic), and wrote out the equations (symbolic). Finally, the penny dropped: those spectator ions they’d crossed out were the same ones that seemingly floated around unchanged while others formed a precipitate in the test tubes.
Importantly, none of the individual ideas were new, but when I explicitly linked the processes at different levels, it helped my students understand the conceptual connections between them.
I know that modelling thinking is a powerful thing for teachers to do, so when I read about a teacher using a worksheet to demonstrate links between conceptual levels, I decided to use a similar worksheet with my own students.
Download this
A template for a worksheet linking conceptual levels. Download
A template for a worksheet linking conceptual levels from the Education in Chemistry website: rsc.li/2PpkbYO
It can be used for:
1. Scaffolding explanations
As you teach ideas or explain concepts, show students what it ‘looks like’ on different levels, eg pictures of water changing state (macroscopic), particle diagrams (sub-microscopic) and state symbols (symbolic) on one sheet can be compared in future to a similar sheet incorporating a heating curve.
2. Illustrating concepts behind practicals
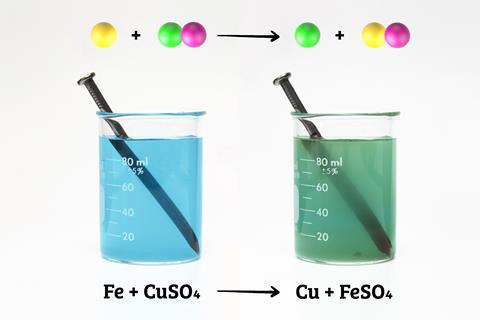
For example, colour changes in displacement reactions (macroscopic) alongside diagrams of ions in solution and metal particles forming a coating (sub-microscopic) with relevant equations (symbolic).
3. Eliciting misconceptions
For example, asking students to describe what’s happening at each conceptual level when chlorine, sodium chloride and sodium change state. You’ll find out how well they understand key similarities and differences.
4. Modelling thinking
For example, when teaching bond energy calculations, explicitly linking pictures of molecules and bonds (sub-microscopic) with the calculations you choose (symbolic), and the observations you’d make (macroscopic).
5. Explaining concepts behind rules
For example, when teaching the formula to calculate concentration (symbolic), explicitly link it to observations (macroscopic) and particle diagrams (sub-microscopic) that will frame later thinking during titration calculations.
Importantly, this shouldn’t be a one-off process. It’s unlikely you’d teach any of these ideas without considering different conceptual levels in your teaching and explanations. But by modelling your own thought processes, and making connections that are second nature to you explicit to your students, you should help them link ideas independently.
Downloads
Johnstone's triangle worksheet
Word, Size 85.72 kbJohnstone's triangle worksheet
PDF, Size 38.46 kb









No comments yet