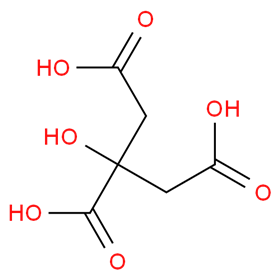
When you squeeze lemon juice over your pancakes this Shrove Tuesday, you will be using citric acid – 2-hydroxypropane-1,2,3-tricarboxylic acid. This molecule has a host of applications, making it quite a magnificent molecule

Citric acid gives citrus fruits – oranges, lemons and limes – their bitter taste. The taste receptors on your tongue detect ‘sour’ when they pick up hydronium ions (H3O+), formed when H+ ions react with water. Citric acid has four available H+ ions. This bitter taste means citric acid is used as an additive in soft drinks. As well as improving the flavour, citric acid (mixed with its salt, sodium citrate) also acts as a buffer, helping to control the pH. And because it is soluble, this magnificent molecule will even dissolve in concentrated syrups.
Citric acid can also help detergents work better in ‘hard’ water, which contains minerals such as calcium carbonate and magnesium sulfate. The calcium and magnesium ions bond to the negatively charged ends of detergent molecules, stopping them from forming a lather. Citric acid is sometimes added to detergents because when it loses its H+ ions, the resulting citrate ion forms a strong bond to these metal ions, letting the detergent molecules get on with their job.
Ever noticed when you add lemon juice to a fruit salad, the apples and bananas don’t go brown straight away? The citric acid in the juice makes the pH too low for the enzymes in fruit to react with oxygen, which is the cause of food discolouring.
Originally published in InfoChem









No comments yet