Eric Scerri explores the composition of group 3 in the periodic table, and why it should be updated
![Group 3 myths i stock 000005756628 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/480xAny/5/7/9/113579_group3myths_istock_000005756628_300tb1.jpg)
The fifth idea in our series is the view that group 3 of the periodic table consists of Sc, Y, La and Ac. There is now enough evidence to show this is incorrect. In 1982, an article in the Journal of Chemical Education argued group 3 should instead consist of Sc, Y, Lu and Lr.1 Some textbook authors have taken this up, but the majority seem reluctant.2
Early groupings
The author of the 1982 article was not the first to group the elements of group 3 in this way. Many earlier periodic tables had the same grouping. Physicists in the 1950s and 60s suggested lutetium should be in group 3 rather than the final member of the first row of the f-block.3
The 1982 article suggested an incorrect assignment of the ytterbium atom’s electronic configuration led to the placement of lanthanum, rather than lutetium, directly below yttrium in group 3. Ytterbium was assumed to have a configuration of [Xe] 4f135d16s2 and lutetium’s was assumed to be [Xe] 4f145d16s2. As a result, the differentiating 4f electron in lutetium implied that it should be the last member of the first row of the f-block. However, spectroscopic measurements revealed both elements possessed 14 f-electrons.4 This meant both had an equal claim to be the last member of the first row of the f-block. If ytterbium occupied this position, the subsequent element, lutetium, would have to be the first element in the third row of the transition metals.
Similarly, because lanthanum and lutetium have the configurations [Xe] 5d16s2 and [Xe] 4f14 5d1 6s2 respectively, both could occupy the first place in the third row of the transition metal series below scandium and yttrium, in principle. If lutetium occupies this position, following its removal from the f-block as discussed above, lanthanum becomes the first member of the f-block elements. Some object to this placement because lanthanum lacks f-orbital electrons. But, this is not an anomaly since more serious cases are tolerated. Thorium’s atom possesses no f-orbital electrons, yet there is no doubt that it belongs in the f-block.
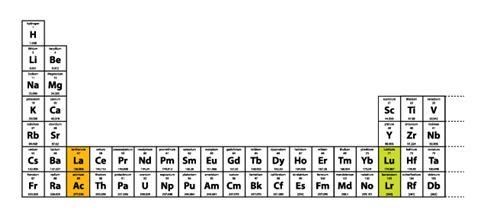
Figure 1 Long-form periodic table showing all elements in order of increasing atomic number
Electronic configurations are ultimately approximations to what is more fundamentally described as a superposition of several closely lying configurations. An element’s atoms do not require a particular kind of differentiating electron for it to belong to the corresponding block of the periodic table. Helium, whose atoms have a differentiating s-orbital electron, is almost always placed in the p-block.5
Long-form periodic tables
There is a different reason why placing lutetium and lawrencium, rather than lanthanum and actinium, in group 3 is the better option.6 If we incorporate either lutetium and lawrencium or lanthanum and actinium into group 3 of the long-form periodic table, only the first placement is consistent because it results in a continuously increasing sequence of atomic numbers (Figure 1). Conversely, incorporating lanthanum and actinium into group 3 of the long-form table results in two glaring anomalies in terms of sequences of increasing atomic numbers (Figure 2).
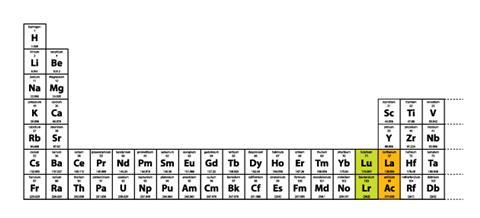
Figure 2 Long-form periodic table showing alternative placements of Lu and Lr. The continuous sequence of atomic numbers shown in Figure 1 is lost – lanthanum, element 57, appears between elements 71 and 72, while actinium, 89, appears between elements 103 and 104
A fly in the ointment
For me, this is a virtually conclusive argument in favour of group 3 consisting of Sc, Y, Lu and Lr. The only fly in the ointment is a third possibility, but this involves an awkward sub-division of the d-block elements (Figure 3). As such, it is not a fatal objection to the group 3 assignment that is being proposed in this article.
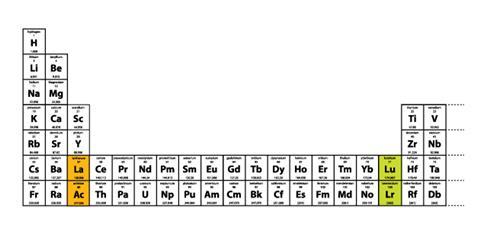
Figure 3 Third option for presenting the long-form periodic table in which the d-block is split into two uneven portions of one and nine groups
A recent article in Nature magazine was reported in the popular science press as having contributed to the resolution of the group 3 conundrum.7 Unfortunately, various members of its large team of authors appear to have used the same data to arrive at opposite conclusions. This highlights the need for a categorical means of settling the issue, which I claim to have provided in my article in Chemistry International.8 In any case, it is high time that the idea of group 3 consisting of Sc, Y, La and Ac is abandoned.
References
- W B Jensen, J. Chem. Educ., 1982, 59, 634 (DOI: 10.1021/ed059p634)
- D W Oxtoby, H P Gillis and A Campion, Principles of Modern Chemistry, Brooks/Cole, 7th edn, 2012, p. 71. However, on the inside cover, the table shows lanthanum and actinium in group 3.
- L Landau and E M Lifshitz, Quantum mechanics, Pergamon, 1959, p. 245; D C Hamilton and M A Jensen, Phys. Rev. Lett., 1963, 11, 205 (DOI: 10.1103/PhysRevLett.11.205); D C Hamilton, Am. J. Phys., 1965, 33, 637 (DOI: 10.1119/1.1972042)
- Jensen implies that this reassignment of the configuration of ytterbium has been a ‘recent’ change. As a matter of fact, it was first made in 1937.
- This is not the case in the Janet or left-step periodic table, however, in which helium is placed in the s-block.
- E Scerri, A very short introduction to the periodic table, Oxford University Press, 2008, ch. 10
- T K Sato et al., Nature, 2015, 520, 209 (DOI: 10.1038/nature14342)
- The proposal was first made in E R Scerri, Chem. Int., July–August 2012, 34, 28
Five ideas in chemical education that must die
- 1
- 2
- 3
- 4
- 5
- 6
![Group 3 myths i stock 000005756628 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/100x67/5/7/9/113579_group3myths_istock_000005756628_300tb1.jpg) Currently reading
Currently readingGroup 3





![I stock 000036213086 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/100x67/5/7/8/113578_istock_000036213086_300tb1.jpg)
![Shutterstock 195864272 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/100x67/6/3/8/113638_shutterstock_195864272_300tb1.jpg)





No comments yet