Students observe what happens when oil and water are mixed, and how the results of the experiment change when various substances, including an emulsifier, are added.
Student Sheet
In this practical I will be:
- Carrying out the practical, making careful observations.
- Accurately using key terms such as mixture, emulsion, emulsifier, hydrophilic and hydrophobic.
- Using my observations of the experiments to draw conclusions about the properties of the liquids used in the practical.
- Suggesting ways of creating an emulsion from two non-mixing, based on my observations of the experiment.
Introduction:
You are an ancient Egyptian science-artist, and you have heard of an up-and-coming artist whose new paints are causing quite a stir. Apparently he has been using lots of different minerals and other materials to create paints of amazing colours. However, when you tried to use these minerals in the past, you were unable to make a useable paint by just adding water. Apparently he adds a secret ingredient to his paint, and according to rumours it has something to do with chicken eggs. Like all good science-artists, you decide to investigate further…
Equipment:
- 6 small screw top bottles (100 cm3) or test tubes and bungs
- 5 disposable (teat) pipettes – 1 for the water, 1 for the oil, 1 for the detergent 1 for the egg white and 1 for the egg yolk (it may be easier to use a spoon for the egg yolk)
- 3 teaspoons (or plastic disposable spoons)
- 2 cm3 any cooking oil
- 2 cm3 good quality detergent
- 10 g sugar
- 10 g flour
- 10 g mustard powder
- 1 egg
- 2 bowls (or 100 cm3 glass beakers)
- Egg yolk separator (or separate using the egg shell)
- Plastic disposable gloves
Method:
Part 1
- Using a (teat) pipette put about 2 cm3 of oil into a screw top bottle (or test tube).
- Using another (teat) pipette add about 2 cm3 of water.
- Put the top on tight or bung in.
- Shake the mixture in the bottle/test tube for about a minute.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Remove the screw top/(bung and leave the mixture to stand for around 5 to 10 minutes.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Repeat steps 1 and 2 with a fresh bottle or test tube.
- Add 2 cm3 of detergent to the mixture of oil and water.
- Put the screw top on/bung in tight.
- Shake the mixture in the bottle/test tube for about a minute.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Remove the screw top/bung and leave the mixture to stand for
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Repeat steps 1 and 2 with a fresh bottle/test tube.
- Add half a teaspoon of sugar to the mixture of oil and water.
- Put the screw top on/bung in tight.
- Shake the mixture in the bottle/test tube for about a minute.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Remove the screw top (bung) and leave the mixture to stand for around 5 to 10 minutes.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Repeat steps 1 and 2 with a fresh bottle (test tube).
- Add half a teaspoon of flour to the mixture of oil and water.
- Put the screw top (bung) on tight.
- Shake the mixture in the bottle (test tube) for about a minute.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Remove the screw top (bung) and leave the mixture to stand for around 5 to 10 minutes.
- Record what you see by taking a photograph, draw a picture or write a sentence.
Part 2
- Repeat steps 1 and 2 with a fresh bottle (test tube).
- Add half a teaspoon of mustard to the mixture of oil and water.
- Put the screw top (bung) on tight.
- Shake the mixture in the bottle (test tube) for about a minute.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Remove the screw top (bung) and leave the mixture to stand for around 5 to 10 minutes.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Take the egg and break it into the egg yolk separator above a bowl. (Or separate the yolk from the white by passing the egg between the egg shells. The yolk will drain into the bowl or beaker while the egg yolk will stay in the shell you are holding. When separated place the egg yolk into a different bowl or beaker from the egg white.
- Let the egg white drain through into the bowl
- Put the egg yolk into another bowl
- Repeat steps 1 and 2 with a fresh bottle (test tube).
- Add about 2 cm3 of egg white to the mixture of oil and water.
- Put the screw top (bung) on tight.
- Shake the mixture in the bottle (test tube) for about a minute.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Remove the screw top (bung) and leave the mixture to stand for around 5 to 10 minutes.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Repeat steps 1 and 2 with a fresh bottle (test tube).
- Add about 2 cm3 of egg yolk to the mixture of oil and water. (You may find it easier to use a spoon rather than a pipette for this).
- Put the screw top (bung) on tight.
- Shake the mixture in the bottle (test tube) for about a minute.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Remove the screw top (bung) and leave the mixture to stand for around 5 to 10 minutes.
- Record what you see by taking a photograph, draw a picture or write a sentence.
- Discuss the results and work out which out of detergent, sugar, flour, mustard, egg white and egg yolk, worked as an emulsifier.
Theory:
A range of substances commonly found in the kitchen can stabilise an oil and water emulsion (a mixture of one liquid dispersed in another liquid). Stabilise means the two liquids do not separate but remain mixed as a mixture called colloid. Colloids such as these are often found in foods. In paints some substances are added to allow the oil, pigment and other liquids to blend into an emulsion, hence the name emulsion paint.
An emulsifier is a substance that stabilises an emulsion. Detergent, egg yolk and mustard are emulsifiers, the others are not. Students may observe colloidal mixtures in the other bottles, but they are not oil and water emulsions and two separate layers should be clearly seen.
The oil and water mixed much better when the egg yolk was added. The protein lecithin, which is in the egg yolk, acts as an emulsifying agent. Emulsifying agents have regions on the molecule that act as a bridge between the oil and the water. The lecithin molecules in the egg yolk form a layer around the oil droplets and prevent the tiny oil droplets from coming together to make a separate layer. Because the oil is still separate from the water even with the egg yolk present, it cannot be a solution. The emulsion created is a dispersion of oil inside the water, with the egg yolk acting as an emulsifier.
Deeper level chemistry for secondary pupils
Water (H2O) is a polar molecule meaning it has positively and negatively charged ends. This polarity is created because the electrons are attracted to the oxygen (O) atom (blue in diagram) of the molecule more than the hydrogen (H) atom (red in diagram). The density of electrons makes the oxygen slightly negative while the hydrogen will be slightly positive (see below).
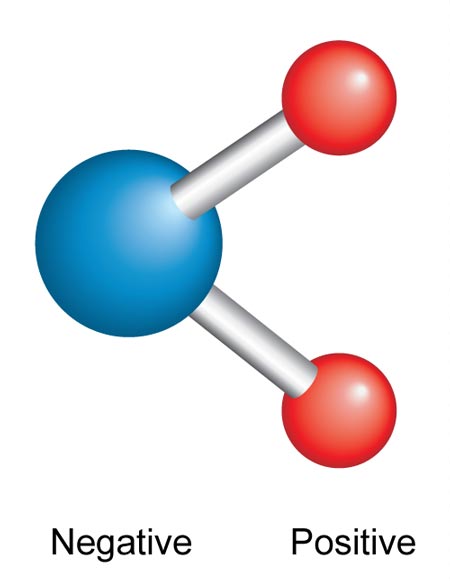
The polarity means that water molecules can attract each other. When salt (sodium chloride) is put into water, it breaks down to form charged ions: a positively charged sodium ion (Na+) and a negatively charge chloride ion (Cl−). The water is attracted to the ions and surrounds them so the ions are mixed evenly throughout the water. We call this dissolving. So ions or molecules with a slight or strong charge can dissolve in water.
Oil does not have a polarity so it is not attracted to the water. Oil molecules will clump together. They are known as hydrophobic molecules from the Greek hydro, meaning water; phobos, meaning fear. Sugar does dissolve in water because it has a slight negative charge that is attracted to the water. Sugar is known as a hydrophilic molecule from the Greek hydro meaning water; philia, meaning love.
Teacher and Technician Sheet
In this practical students will:
- Carry out the practical, making careful observations.
- Accurately use key terms such as mixture, emulsion, emulsifier, hydrophilic and hydrophobic.
- Use their observations to draw conclusions about the properties of the liquids used in the practical.
- Suggesting ways of creating an emulsion from two non-mixing, based on observations of the experiment.
Introduction:
This experiment can easily be done in a kitchen as ‘making a salad dressing’ using oil and vinegar rather than oil and water. You can taste the resulting mixtures as well as observing them. If you do this, do not taste the ones containing raw egg.
Students should never be encouraged to put anything in their mouths when carrying out experiments.
It is strongly advised students should not eat or drink anything in a school laboratory.
This practical can take a long time so the teacher can either split the class into two and one half do the first part and the other half do the second half then the students come together to compare their results.
Time can also be saved by having the oil and water in the bottles for the students.
A mixture of oil and water usually separates quickly, but a range of substances act as emulsifiers.
Curriculum range:
This practical can be carried out by primary and younger secondary age students. This practical links with:
- reporting on findings from enquiries, including oral and written explanations, displays or presentations of results and conclusions;
- using straightforward scientific evidence to answer questions or to support their findings;
- compare and group together everyday materials on the basis of their properties;
- know that some materials will mix, while others will not;
- know that liquids that do not mix can be turned into emulsions;
- build a more systematic understanding of materials by exploring and comparing the properties of a broad range of materials.
Hazard warnings:
Due to salmonella risk, handling raw egg should be kept to a minimum, so provide plastic gloves and use disposable plastic pipettes (or disposable plastic spoons) for students to transfer the egg to the screw top bottles (test tubes).
Equipment:
Per group:
- 6 small screw top bottles (100 cm3) or test tubes and bungs
- 5 disposable teat pipettes – 1 for the water, 1 for the oil, 1 for the detergent 1 for the egg white and 1 for the egg yolk (it may be easier to use a spoon for the egg yolk)
- 3 teaspoons (or disposable plastic spoons)
- 2 cm3 any cooking oil
- 2 cm3 good quality washing up liquid
- 10 g sugar
- 10 g flour
- 10 g mustard powder
- 1 egg
- 2 bowls (or 100 cm3 glass beakers)
- Egg yolk separator (or separate using the egg shell)
- Plastic disposable gloves
Technical notes:
- Using small screw top bottles means it is easier to see what is going on and much easier to clean up. The bottles must be very clean and must not be contaminated with detergent. Test tubes and bungs can also be used.
- Corn oil is a good oil to use because of its colour, which is easier to see.
- Use a good quality detergent because cheaper detergents do not usually work very well.
- Colman’s mustard powder is good and the powder lasts longer than ordinary mustard.
- Use fresh eggs. It is easier to separate these, and if necessary you can buy an egg yolk separator. Try to make sure no yolk contaminates the white – the other way round is less important.
Results:
Students should be able to observe:
|
Water and oil |
Water mixes into oil making a cloudy mixture with a thicker consistency. After 5-10 minutes the oil and water separate into two distinct layers with water on the bottom layer and oil on the top. |
|
Adding detergent
N.B. |
Produces a cloudy mixture with a thin consistency and layer of foam on top. After 5–10 minutes this produces a liquid bottom layer and some foam above that indicating an emulsifier.
This can give a false reading and separate into distinct layers if a weak detergent (or not enough) is used. |
|
Adding sugar |
Mixes into a thicker paste consistency then separates after 5–10 minutes into two separate layers. |
|
Adding flour |
Mixes into a milky substance with some oil globules visible in the liquid. After 5–10 minutes the solid does not dissolve but settles to the bottom, with two distinct layers of liquid and then oil as the top layer. |
|
Adding mustard |
This mixes in well producing a yellow liquid with powder particles visible on the walls of the bottle. After 5–10 minutes nothing has changed and the solution remains mixed well. This should indicate to the student that the mustard is an emulsifier. |
|
Adding egg white |
Mixes into a cloudy gelatinous substance with no visible oil. After 5–10 minutes there are two (less distinct) layers. The bottom layer is a darker cream than the top with the top layer whiter and slightly foamy. |
|
Adding egg yolk |
This mixes in completely producing a cloudy yellow liquid that is still the same after 5–10 minutes, indicating an emulsifier. |
Overall, this experiment is easy to provide and carry out with results that support the chemistry notes and consolidate the practical learning experience.
Downloads
Making an oil-water emulsion: student sheet
Experiment | PDF, Size 0.19 mbMaking an oil-water emulsion: teacher sheet
Experiment | PDF, Size 87.08 kb

















