- Home
- I am a …
- Resources
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- Literacy in science teaching
- More …
- Climate change and sustainability
- Alchemy
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Collections
- Education in Chemistry
- Teach Chemistry
- Events
- Teacher PD
- Enrichment
- Our work
- More navigation items
Structure and bonding post-16
Classroom resources featuring activities from our Structure and bonding post-16 professional development course for teachers
This collection is most valuable to those who have attended this course and wish to put into practice with their students some of the ideas and activities presented as part of that event. Please note that this list is not exhaustive; not all trainer activities have a corresponding classroom resource. In some circumstances there is variation between the training resource and classroom resource.
Chemical misconceptions II: Spot the bonding | 16–18 years
This activity explores learners knowledge of different bonding types through 18 diagrams
Chemical misconceptions II: Interactions
Support students to explore more forms of interactions between bonds, identify, and explain them.
Teachers TV: chemistry exciting elements
This Teachers TV video demonstrates exciting experiments around the topic of elements, such as reacting bromine with hydrogen and the displacement of halogens.
Making silicon and silanes from sand
Create silicon in your classroom using just sand and magnesium. This exothermic practical will show learners the nuances of heat based reactions and how to perform them safely. Kit list and safety instructions included.
Chemical misconceptions II: Ionisation energy
Use this activity as a revision of the topic, or a reinforcement of learner’s knowledge base, upholding what they know about ionisation energy.
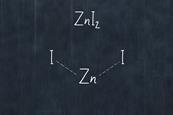
Exothermic redox reaction of zinc with iodine
Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This can be reversed using electrolysis to decompose the compound.
Jets of liquids: the effect of charged plastic on water
Polar covalent bonds, and their affects on water are explored in this open-ended problem.
The thermal properties of water
Explore water’s boiling point, specific heat capacity and thermal conductivity in this demonstration. Includes kit list and safety instructions.
Formation of TCP (2,4,6-trichlorohydroxybenzene)
Delve into preparing TCP by reacting hydroxybenzene (phenol) with chlorine gas, and create this distinctive smelling compound.