All Chemistry for the Gifted and Talented articles
-
 Resource
ResourceSolutions | Stretch and challenge | 11–14 years
Challenge learners to apply logical reasoning and their knowldge of particle model to explain experimental observations
-
 Extension
ExtensionBoiling point: a surprising measurement | Stretch and challenge | 11–14 years
Develop critical thinking skills by guiding learners to evaluate possible explanations for an unexpected experimental result
-
 Extension
ExtensionBonding models | Stretch and challenge | 14–16 years
Challenge learners to explore alternative models, using plasticine and cocktail sticks
-
 Extension
ExtensionIonic bonding and electron transfer | Stretch and challenge | 14–16 years
Learners evaluate and discuss ideas about ionic bonding, the formation of ions and energetics
-
 Resource
ResourceRates and equilibria
This activity demonstrates the links between the topics of rates of reaction and the equilibrium law. It provides students with an explanation of the equilibrium law and helps them explain why Le Chatelier’s principle works for temperature, concentration and pressure.
-
 Resource
ResourceOrganising your understanding
Three activities that progressively stretch learners’ understanding of these key topics using Venn-like diagrams to organise information
-

-
 Resource
ResourceSwimming pool chemistry | 16–18 years
Synoptic questions explore structure and bonding, equilibrium and spectrometry and spectroscopy in the context of swimming pools
-
 Resource
ResourceShapes of molecules and ions
This activity draws some extra concepts and mathematical skills into the discussion of molecular shape. A distinction between geometry around the central atom and the shape of the molecule is made.
-
 Resource
ResourceThe second law of thermodynamics
This activity aims to introduce the topic in a way that uses the students’ synthesis skills to piece together several pieces of information.
-
 Resource
ResourceOxidation numbers
This activity introduces oxidation numbers by giving a conceptual foundation for them in terms of electron accounting and polar bonds. It then shows how the model used so far needs refining.
-
 Resource
ResourceOrganic reaction maps
This activity encourages the use of mind maps to organise information. It also highlights where oxidation and reduction are involved in transformations between functional groups.
-
 Resource
ResourceMixing drinks
The activity uses two methods to develop metacognition. First, students are asked to solve a problem and then reflect on the thinking styles that they used. In the other method students discuss four modelled thinking styles of fictional students.
-
 Resource
ResourceOdd one out (organic)
This activity should encourage a rapid consideration of the range of concepts met in organic chemistry.
-
 Resource
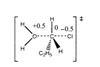
ResourceFormal charge
This activity introduces formal charge – a useful tool which otherwise might not be taught. The formal charge model treats bonds as pure covalent, in contrast to the oxidation state model which treats bonds as ionic.
-
 Resource
ResourceEntropy and equilibrium
This activity shows the students the fundamental link between entropy and equilibrium and increases students’ understanding of scientific models. It highlights the importance of mathematical descriptions in physical chemistry.
-
 Resource
ResourceCurly arrows and stereoselectivity
This activity develops the use of curly arrows. If you have some very able students in a group, then there is an opportunity for differentiation by giving them a chance to draw curly arrow mechanisms for whatever reactions they meet.
-
 Resource
ResourceCovalent bonding
This activity seeks to develop an understanding of covalent bonding in terms of energetic stability rather than full shells.
-
 Resource
ResourceA new kind of alchemy
This presents some cutting edge research for post-16 students in a context that they can appreciate. It shows the students there are still big ideas to be explored in chemistry and should promote research as a career choice.
-
 Resource
ResourceTrends in reactivity in the periodic table
This could be used to follow up some work on the periodic table where the trends in reactivity in groups 1 and 7 have been identified. It can be used as a differentiated activity for the more able students within a group.











