Morag Easson explains how to neutralise student difficulties
The chemistry of acids and bases can appear wonderfully colourful, but occasionally mysterious to some students. Their understanding can be hindered by confusing language and a lack of appreciation of what exactly is happening in solution at the particle level. Getting to grips with the mathematical manipulations and logarithms required can lead some to perceive acids and bases as a tricky area of chemistry. The need to apply equilibria concepts or understand indicators at an advanced level can add to students’ worries.

Research describes what students find difficult about acid–base chemistry.1 This is reflected in the approaches outlined here to support students in their learning.
Common difficulties
Acids and bases are all around us, including a diverse array of food, drink and domestic products, providing many opportunities to explore real contexts and applications of chemistry. However, the over-exaggerated portrayal of acids in the media as highly corrosive substances that ‘eat away’ materials in their path is likely to be familiar to students and can be unhelpful. Indeed, some bases are even more corrosive, and yet bases don’t share the reputation of their acidic counterparts.
The corrosive nature of alkalis could be highlighted by posing a question such as, 'Which are the most dangerous, acids or alkalis?' as a topic for discussion.
Many teachers introduce the idea of acids and pH with a simple and robust practical activity testing the pH of everyday items with pH paper or universal indicator. Sufficient examples of bases should be included to ensure their familiarity from the outset. Students can be encouraged to test domestic items, from fruit juices to cleaning products.
The pH scale poses its own challenges. The number scale can, at first, appear ‘back to front’, as the lower the number the more acidic the solution. However, students usually accept this concept quickly and are able to use the scale to measure pH. They may need reminding that the pH scale ranges from below 0 and above 14, even though most examples met in school lie between the two.
Zooming in on neutralisation
Neutralisation reactions are very useful and common in daily life. For example, toothpaste is alkaline in order to neutralise acids in the mouth that would otherwise damage our teeth. The underlying chemistry is important in explaining the behaviour of acidic solutions. Before launching into these concepts, it is essential students have a basic understanding of what happens when ionic substances are added to water.
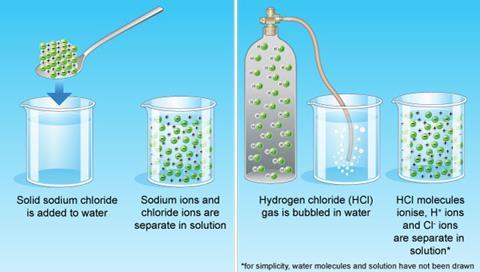
Would your students be able to explain or sketch what happens when an ionic solid or gas (see figure 1) is added to water?
Some students hold the notion that a base somehow halts the acidic behaviour of an acid. They need to appreciate neutralisation is the chemical reaction of an acid and a base. Such difficulties rationalising the behaviour of acids can be addressed by considering the particle nature of solutions of acids and alkalis and the underlying reaction as hydrogen ions react with hydroxide ions.2
One possible approach, measuring the temperature change during neutralisation, shows the reaction gives out heat energy as the net result of bonds breaking and new bonds forming. A thermometric titration between a strong acid and a strong alkali involves measuring the temperature and pH of the resulting solution after every addition of reagent.3 Plotting the values shows the maximum temperature occurs at the neutralisation point.
Making salts is a worthwhile activity that can also help students to explore the concept of neutralisation. For example, the preparation of stunning blue copper sulfate crystals from the reaction of copper oxide and sulfuric acid, on a small scale,4 is very satisfying and deepens students’ understanding when the reasons for each practical step are absolutely clear, eg why they are adding the solid reagent in excess.
We often introduce simple salts and how to name them early on in our studies of neutralisation reactions. Students’ interest can be sustained with an approach that includes some mind games such as Gridlocks5 or card sorts to challenge and motivate them. Writing simplified ionic equations that highlight the ions taking part in the reaction can also help students to focus on the underlying reaction.
Confusing language
As in many areas of chemistry, language matters. The classic confusion regarding acids and bases is to mix up the terms strong and weak with concentrated and dilute. They have entirely different meanings in this context. Strong/weak refers to the degree of dissociation, while concentration is a measure of the amount of solute in a solvent. Perhaps our own everyday language can share the blame for interchanging these words. How often have you heard someone ask for a ‘weak cup of tea’ or a ‘strong cup of coffee’?
Approaches and activities to help understand weak/strong
- Many teachers encourage practical activities showing differences in pH, conductivity and reactivity (eg magnesium or marble chip) between hydrochloric acid (strong) and ethanoic acid (weak).
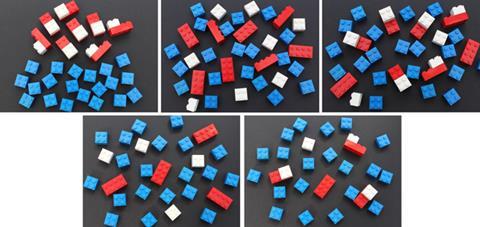
- Keith Taber’s Acid strength paper activity provides a useful tool to check or reinforce understanding at the particle level with older students.6 This may be enhanced by making physical models, eg with Lego bricks (figures 3–7).7
- The PhET acid–base online simulation is great for visualising particles and the relative numbers of particles in solution.8
- Students can make sequential 10-fold dilutions of equimolar solutions of a strong acid and a weak acid. Measuring the pH at each dilution will show the pH changes by 1 pH unit for every 10-fold dilution for the strong acid but only by 0.5 of a pH unit for the weak acid. A similar comparison can be made with strong/weak bases.
How about the words ‘base’, for a substance that reacts with acids, and ‘alkali’, used to describe a water-soluble base? The difference needs to be made explicit so as not to leave students confused.
In post-16 studies, what exactly does ‘neutral’ mean? Neutral is often inadequately defined as pH 7. Neutral occurs when the hydrogen ion concentration is equal to the concentration of hydroxide ions in aqueous solution. This may or may not be pH 7 as the equilibrium is temperature dependent, thus the pH (a measure of H+ ion concentration) varies accordingly.9
Approaches and activities to help understand ‘neutral’
- Consider how terms are presented to students and used at different stages in school. Like many concepts in chemistry, the meaning of ‘neutral’ appears to change or be refined as students progress. In pre-16 studies, it is acceptable to consider neutral as pH 7 because students do not yet have the necessary knowledge and understanding of other underlying chemical concepts – eg equilibria.
- Practical determination of titration curves involving weak acids shows the equivalence point is not 7. Using dataloggers can provide lots more example data.
- Look at experimental data collected at different temperatures and explain trends in terms of the equilibrium, H2 O(l) + H2 O(l) ⇌ H3 O+ (aq) + OH– (aq) and which way it shifts. For example, a shift to a higher concentration of H3 O+ ions gives a lower pH value.
- In advanced studies, the class could be given a lateral thinking problem to discuss – for example, 'a neutral salt solution is pH 6.5' – in order to get students to critically engage with the topic and explore their own ideas.
The pH scale and maths
Logarithms, in particular, can look scary. Demystifying logs by explaining that we use them to make powers of ten more manageable; showing that working with logs boils down to simple addition and subtraction which is easier to handle; and practising which buttons to press on the calculator, can be beneficial. There are some good sources of help,10 including the standard form topic on the freely available online CPD for Teachers course, Maths Skills.11
Morag Easson is a Royal Society of Chemistry trainer and independent science education consultant
References
- From V Kind, Beyond appearances: students’ misconceptions about basic chemical ideas (2nd ed.). Royal Society of Chemistry, 2004
- Hydrogen ions in aqueous solution are associated with water molecules, known as hydronium ions, oxonium ions or hydroxonium ions, and written as H3O+. The use of H+ is accepted as simplified notation.
- Thermometric titration
- Preparation of copper sulfate crystals
- Gridlocks
- Acid strength, from K Taber, Chemical misconceptions, diagnosis and cure (vol. 2). Royal Society of Chemistry, 2002
- Models show the white bricks as H+, and these may be joined to blue bricks (water molecules) to better represent H3O+ when the acid ionises.
- PhET simulation
- Five ideas in chemical education that must die
- D Warren, Sch. Sci. Rev., 2016, 360, 37 (www.ase.org.uk)
- Maths skills online CPD course









2 readers' comments