Use these guiding questions and context to open up the hidden world of atmospheric chemistry for your 14–16 learners
The effects of human activity on our environment are rarely far from the news. Teaching atmospheric chemistry allows us to show 14–16 learners chemistry in a relevant real-life context. But it can be tempting for them to focus on memorising the information required, rather than thinking about the chemistry and physics involved. This article provides some background context and guiding questions you can use to develop your learners’ understanding.
Where does a planet’s atmosphere come from?
It is composed of substances with a relatively low boiling point compared to the planet’s surface temperature. These substances may have arrived from space on colliding meteorites or comets. More likely, the substances were formed within volcanoes due to high-temperature thermal decomposition of the rocks. Learners will be aware of the decomposition of carbonates to form carbon dioxide, for example CaCO3(s) ⟶ CaO(s) + CO2(g). Another source of atmospheric gases is radioactive decay. Argon – the third most abundant gas in the Earth’s atmosphere – is produced by positron decay of 40K.
The effects of human activity on our environment are rarely far from the news. Teaching atmospheric chemistry allows us to show 14–16 learners chemistry in a relevant real-life context. But it can be tempting for them to focus on memorising the information required, rather than thinking about the chemistry and physics involved.
What students need to know
This article provides some background context and guiding questions you can use to develop your learners’ understanding.
Where does a planet’s atmosphere come from? It is composed of substances with a relatively low boiling point compared to the planet’s surface temperature. These substances may have arrived from space on colliding meteorites or comets. More likely, the substances were formed within volcanoes due to high-temperature thermal decomposition of the rocks. Learners will be aware of the decomposition of carbonates to form carbon dioxide, for example CaCO3(s) ⟶ CaO(s) + CO2(g). Another source of atmospheric gases is radioactive decay. Argon – the third most abundant gas in the Earth’s atmosphere – is produced by positron decay of 40K.
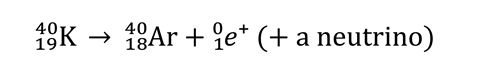
Why is there so little hydrogen and helium in the Earth’s atmosphere?
Learners should know from physics why hydrogen and helium make up over 99.9% of our solar system. Most planets are made up of these two gases (like Jupiter, for example), but why does the Earth’s atmosphere consist of so little of these gases? A simple answer is that the Earth’s gravity isn’t strong enough.
Why is there so little hydrogen and helium in the Earth’s atmosphere? Learners should know from physics why hydrogen and helium make up over 99.9% of our solar system. Most planets are composed of these two gases (like Jupiter, for example), but why does the Earth’s atmosphere consist of so little of these gases? A simple answer is that the Earth’s gravity isn’t strong enough.
For an explanation that will support post-16 student understanding, explain that molecules of all gases will have the same average kinetic energy at a given temperature. Since kinetic energy is proportional to mass multiplied by velocity squared (KE = ½ m v2), lighter molecules, such as hydrogen and helium, will move faster. This allows them to exceed the escape velocity of the Earth, which is just over 11,000 m s–1.
International bans on CFCs and other ozone-depleting compounds have allowed the ozone layer to start to recover
What about methane and ammonia?
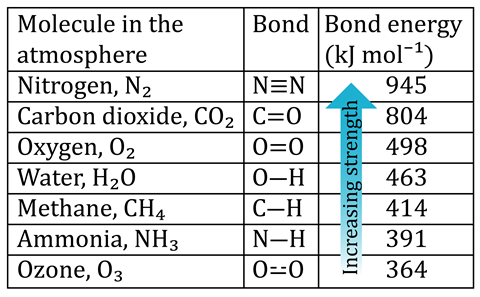
The Earth is continually bombarded by ultraviolet light from the sun. And ultraviolet light can be divided into three ranges, known as UVA, UVB and UVC, depending on the wavelength. The energy of the photons is inversely proportional to the wavelength.
What about methane and ammonia? The Earth is continually bombarded by ultraviolet light from the sun. And ultraviolet light can be divided into three ranges, known as UVA, UVB and UVC, depending on the wavelength. The energy of the photons is inversely proportional to the wavelength.
The bond energies of both carbon dioxide and nitrogen are much higher than the most energetic UVC. Therefore, N2 and CO2 are stable in our atmosphere. By contrast, gases like methane and ammonia will break down over time. This effect also explains why the weaker bonds in ozone can absorb UVB and some UVA radiation that would otherwise damage our skin.
Suggestions for teaching
- Atmospheric chemistry is more than a list of chemical facts to learn by rote – learners can study many important concepts through this topic.
- Use a recent news report on air pollution or climate change as a starter to generate interest.
- Use probing questions such as those suggested in this article to develop understanding and make links with prior physics and chemistry topics.
- Check for common misconceptions, such as confusing depletion of ozone with the greenhouse effect.
Why doesn’t the ozone layer disappear?
The rate at which ozone is broken down is matched by the rate at which more ozone forms. This is a good example of a dynamic equilibrium. Oxygen molecules are broken down by UVC radiation into single oxygen atoms.
Why doesn’t the ozone layer disappear? The rate at which ozone is broken down is matched by the rate at which more ozone forms. This is a good example of a dynamic equilibrium (rsc.li/49NGT3p). Oxygen molecules are broken down by UVC radiation into single oxygen atoms.
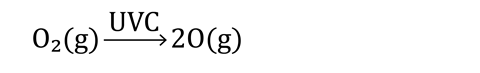
These single atoms then spontaneously combine with more oxygen molecules to continually replace the ozone.
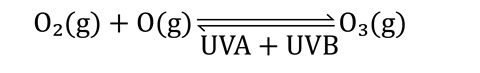
This ozone/oxygen balance was upset in the 20th century. Human activity released huge quantities of chlorine-containing compounds, such as chlorofluorocarbons (CFCs), into the atmosphere. In the upper atmosphere, these compounds are broken down under UVC radiation, releasing chlorine atoms. Learners going on to study post-16 courses will discover how chlorine atoms catalyse the subsequent breakdown of ozone. In recent years, international bans on CFCs and other ozone-depleting compounds have allowed the ozone layer to start to recover.
Why are oxygen and water stable to UV light?
Neither oxygen nor water absorbs UV radiation. Most of the water on Earth has condensed to form the oceans and high-energy UVC radiation cannot reach the oceans. Any oxygen that is broken down in the upper atmosphere is replaced by photosynthesis. This biochemical process helps account for the relatively tiny amount of carbon dioxide in the Earth’s atmosphere. Some carbon dioxide also dissolves in the oceans to form carbonates. If it were not for these processes, our atmosphere would be mainly carbon dioxide, like those on Mars and Venus.
Why are oxygen and water stable to UV light? Neither oxygen nor water absorbs UV radiation. Most of the water on Earth has condensed to form the oceans and high-energy UVC radiation cannot reach the oceans. Any oxygen that is broken down in the upper atmosphere is replaced by photosynthesis. This biochemical process helps account for the relatively tiny amount of carbon dioxide in the Earth’s atmosphere. Some carbon dioxide also dissolves in the oceans to form carbonates. If it were not for these processes, our atmosphere would be mainly carbon dioxide, like those on Mars and Venus.
Resources for your classroom
- Discuss how the Earth’s atmosphere has evolved and spark critical discussions with your learners.
- Inspire your students by reading how Zoë finds ways to minimise the impact of humans on the natural world in her role as an atmospheric chemist.
- Use the building better cities through chemistry resource to evaluate real-time data about atmospheric pollutants with your pupils.
Why do greenhouse gases absorb infrared radiation?
The explanation of the greenhouse effect should be familiar to learners. Carbon dioxide and methane absorb infrared radiation. However, a common misconception is that this effect is somehow connected with the ozone layer. Infrared photons have much lower energies than those of UV radiation. Absorption of IR radiation will not break covalent bonds, but will cause the bonds to bend and stretch for the C–H bonds in methane.
Why do greenhouse gases absorb infrared radiation? The explanation of the greenhouse effect should be familiar to learners. Carbon dioxide and methane absorb infrared radiation. However, a common misconception is that this effect is somehow connected with the ozone layer. Infrared photons have much lower energies than those of UV radiation. Absorption of IR radiation will not break covalent bonds, but will cause the bonds to bend and stretch for the C–H bonds in methane.
During post-16 courses, students learn how to identify particular covalent bonds by using infrared spectroscopy.
Teaching atmospheric chemistry provides an ideal opportunity to revisit a variety of 14–16 topics with your learners, such as thermal decomposition, bond energies, catalysts and reversible reactions. It also provides an excellent foundation for those going on to study the subject at post-16.
Martin Bluemel is a retired head of chemistry, with more than 30 years of classroom experience














1 Reader's comment