Use these teaching approaches to help learners understand polymers and overcome misconceptions
The German chemist Hermann Staudinger first proposed the concept of chain-like macromolecules made by repeatedly joining smaller molecules in 1920. However, natural polymers, such as nucleic acids and polysaccharides, were around for a long time before that. The development of synthetic polymers is just catching up with nature.
Polymers, both natural and synthetic, are successful because they are extremely versatile materials with seemingly endless ways to adapt their properties by changing the monomers, branching the chains or by interacting with other substances.
Download this
Context-based questions, for age range 16–18
Use the resource with your post-16 learners to practise applying their knowledge of polymers to an unfamiliar context.
Download the student worksheet and teacher notes, with answers, from the Education in Chemistry website: rsc.li/4ds8pGq
We are also now increasingly aware of the impact that synthetic polymers have in the natural environment and the challenge of how to dispose of them responsibly. Therefore, a thorough understanding of polymers is an important part of all chemistry courses.
What students need to know
At the post-16 level, learners need to be clear about the distinction between addition and condensation polymerisation. They must be able to recognise the key features of an organic molecule that allow it to polymerise.
Understanding polymerisation reactions
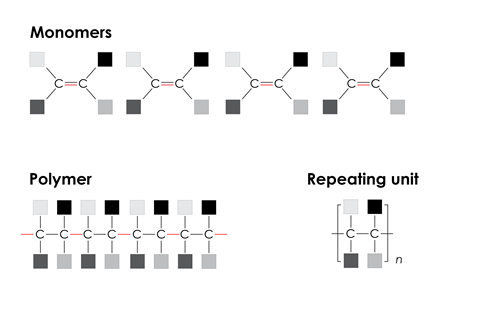
For addition polymerisation, the key feature is a carbon–carbon double bond that can react with those of neighbouring molecules to form a singly-bonded polymer chain.
Remind learners about natural polymers, such as proteins and polysaccharides
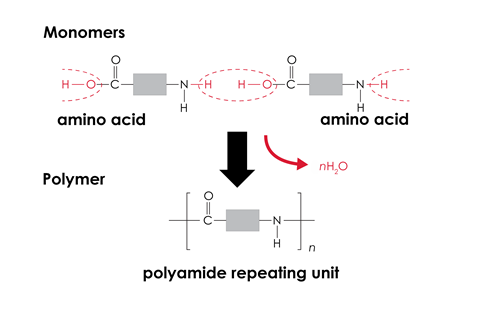
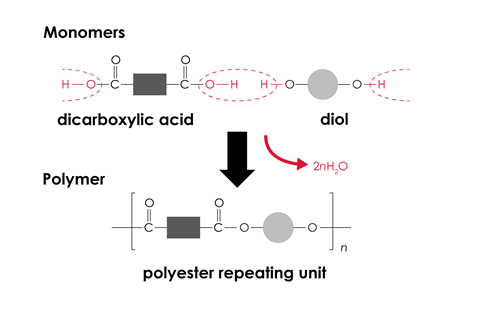
For condensation polymerisation, two functional groups react together with the loss of a small molecule (typically water). These two functional groups may be on each end of the same monomer, or alternatively there may be two different monomers with the same functional group at each end.
Predicting the polymer
For all types of polymerisation, it is important that learners can predict the structure of the polymer that will form from the given monomer(s), or vice versa. They will need to be able to represent the structure of a polymer by showing the displayed formula of the repeating unit.
Common misconceptions
Most learners will already have some knowledge of polymers from their pre-16 courses. However, the somewhat limited understanding in early courses can lead to misconceptions.
Key misconceptions
Learners sometimes think:
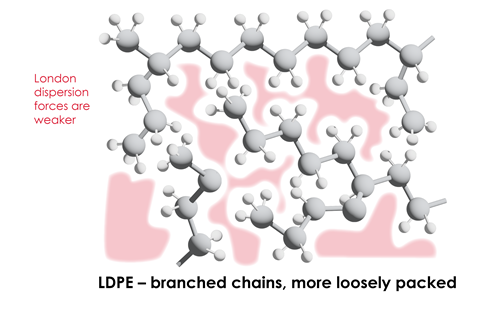
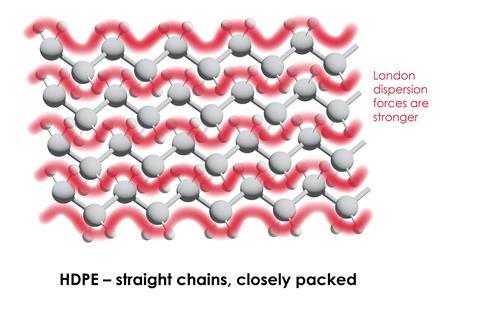
- all polymers are long straight chains. Some polymer chains, for example low density poly(ethene), LDPE, and glycogen, are branched. Other polymers, like urea-methanal, are cross-linked and form giant covalent networks.
- polymers are always formed from identical monomers. The formation of condensation polymers is often from two different monomers, and in RNA and DNA there are four different monomers.
- all condensation polymers form water. The classic nylon rope trick demonstration is a good example of a polymerisation reaction that forms hydrogen chloride as the small molecule.
The emphasis on the role of polymers in everyday plastics can give the impression that all polymers are synthetic and non-biodegradable. You may need to remind learners about natural polymers, such as proteins and polysaccharides.
Learners sometimes think polymers are always formed from identical monomers. The formation of condensation polymers is often from two different monomers, and in RNA and DNA there are four different monomers.
They may also hold the misconception that all condensation polymers form water. The classic nylon rope trick demonstration is a good example of a polymerisation reaction that forms hydrogen chloride as the small molecule.
Some learners struggle to represent the repeating units of condensation polymers. They need to check that the bonds at either end would join to make the correct functional group without any atoms repeated or missing.
Learners sometimes think all polymers are long straight chains. Some polymer chains, for example low density poly(ethene), LDPE, and glycogen, are branched. Other polymers, like urea-methanal, are cross-linked and form giant covalent networks.
Students can also forget that polymers don’t just involve covalent bonding. A polymer’s properties also depend on the intermolecular forces, such as London dispersion forces, between the chains.
Some learners struggle to represent the repeating units of condensation polymers. They need to check that the bonds at either end would join to make the correct functional group without any atoms repeated or missing.
Learners can also forget that polymers don’t just involve covalent bonding. A polymer’s properties also depend on the intermolecular forces, such as London dispersion forces, between the chains.
Ideas for your classroom
An engaging way to reintroduce the topic at this level is to find a current news article that will capture interest, like Slashing emissions with polymer fashion, or The nanoplastic predicament. Some learners will have already met polymerisation, so the Starter for 10 polymers question and answer sheet is a good source of diagnostic questions to test their understanding. You might also want to revisit the addition and condensation polymerisation sections of the RSC Organic chemistry worksheets for 14–16 year-olds.
More resources
- Explore how the properties of polymers depend on their structure and different types of intermolecular bonds using this lesson plan for 16–18 year-old learners.
- Watch this Exhibition chemistry video to see a self-pouring, cuttable liquid polymer. Download the technician notes to do the demonstration in your class.
- Display this poster and use the accompanying resource to test learners’ understanding of addition polymers’ structure.
- Show learners how polymers are used in coatings for wind turbine blades with this video job profile of Maria, a section leader at AkzoNobel.
- Get the poster, fact sheet and classroom activity for teaching condensation polymerisation for 16–18 year-old students.
For a practical demonstration of polymerisation, the formation of polystyrene shows addition polymerisation, whereas for condensation polymerisation it is hard to beat the classic nylon rope trick. There are also everyday examples of polymerisation that learners can see, such as poly(cyanoacrylate) in superglue and forensics, and poly(methyl 2-methylpropenoate) in nail varnishes and paints. These two polymers show a range of functional groups attached to the chain.
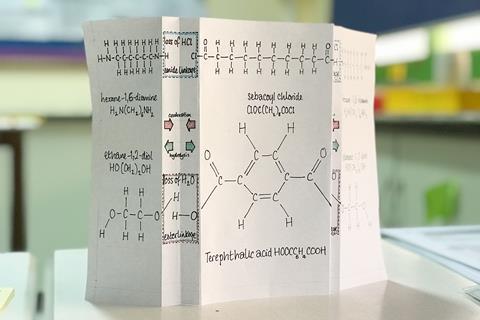
If learners need support with condensation polymers, create foldable paper prompts as described in Faisal Khan’s Fold for victory article as a great way to show the repeating unit. It can be particularly effective if different members of the group make different polymers.
Real-life polymers
Read about the discovery of acrylic and its role in the beauty industry in the Everyday chemistry article, Polish up your knowledge of nail varnish. See also Polished polymers for more detail on nail varnishes.
Finally, people discovered a number of commonly used polymers by accident. Use their stories to provide further interest to your lessons, either as short anecdotes or as a poster activity where each learner researches and presents a particular polymer to the class. See Not all experiments go to plan as well as Sodium poly(acrylate) and PET from the Magnificent molecules series for ideas.
Ideas for your classroom
An engaging way to reintroduce the topic at this level is to find a current news article that will capture interest, like slashing emissions with recyclable polymer fashion or nanoplastics in bottled water. Some learners will have already met polymerisation, so the Starter for 10: polymers question and answer sheet (rsc.li/3JPfsQb) is a good source of diagnostic questions to test their understanding. You might also want to revisit the addition and condensation polymerisation sections of the Organic chemistry worksheets for 14–16 year-olds: rsc.li/3UMRrvc
For a practical demonstration of polymerisation, the formation of polystyrene shows addition polymerisation, whereas for condensation polymerisation it is hard to beat the classic nylon rope trick (rsc.li/4bqDPeC). There are also everyday examples of polymerisation that learners can see, such as poly(cyanoacrylate) in superglue and forensics, and poly(methyl 2-methylpropenoate) in nail varnishes and paints. These two polymers show a range of functional groups attached to the chain. Read about the discovery of acrylic and its role in the beauty industry in the Everyday chemistry article, Polish up your knowledge of nail varnish (rsc.li/3y5k7qh).
If learners need support with condensation polymers, the creation of foldable paper prompts as described in Faisal Khan’s Fold for victory article (rsc/li.3UM3SXY) is a great way to show this. It can be particularly effective if different members of the group make different polymers.
Finally, a number of commonly used polymers were discovered by accident. Use their stories to provide further interest to your lessons, either as short anecdotes or as a poster activity for which each learner researches and presents a particular polymer to the class. See Not all experiments go to plan (rsc.li/3JRjRxN) as well as Sodium poly(acrylate) and PET from the Magnificent molecules (rsc.li/3JNFUp0) series for ideas.
More resources
- Explore how the properties of polymers depend on their structures and different types of intermolecular bonds using this lesson plan for 16–18 year-old learners: rsc.li/3ykX2jv
- Watch this Exhibition chemistry video to see a self-pouring, cuttable liquid polymer. Download the technician notes to do the demonstration in your class: rsc.li/4dL8CEL
- Display this poster and use the accompanying resource to test learners’ understanding of addition polymers’ structure: rsc.li/3wFooAi
- Show learners how polymers are used in coatings for wind turbine blades with this video job profile of Maria, a section leader at AkzoNobel: rsc.li/3V1ndoa
Checking for understanding
Give learners as much opportunity as possible to explore the conversion of monomer to polymer by using whiteboards and models in lessons, as well as exam-style questions to work on. They will discover what works and doesn’t and your feedback will help them see anything they did not spot themselves.
Start with simpler examples and move on to those that contain more complex functional groups as learners become more confident. Make sure that you include examples of condensation polymers with one monomer and with two monomers, and include polyamides, polyesters and polysaccharides to assess understanding of all the common functional groups.
Take-home points
- Teaching polymers post-16 will usually build on some prior knowledge of polymerisation, so diagnose where learners are at before you get too far into the topic.
- Help learners become more aware of the ubiquity of natural and synthetic polymers and add interest to your lessons by using practical demonstrations and stories about their discoveries and everyday applications.
- Give lots of opportunity to practise drawing the repeating unit from given monomers and vice versa. Increase the complexity of the functional groups as learners become more confident.
- Use the topic to reinforce knowledge and understanding of the reactions of organic functional groups whenever possible.
Article and resource written by Martin Bluemel, a retired head of chemistry. Resource produced with support of The Worshipful Company of Horners















No comments yet