Improve your post-16 learners’ understanding of electronegativity and intermolecular forces
Pure covalent bonding and ionic bonding can be considered as opposite ends of a bonding continuum, or spectrum.
In a covalent bond, atoms share pairs of electrons. The covalent bond is the result of two positive nucleuses being held together by their common attraction for the shared pair of electrons. The ionic bond is the electrostatic attraction between positive and negative ions within a crystal lattice.
Download this
Infographic poster, fact sheet and student worksheet exploring the bonding spectrum from pure covalent bonding to ionic bonding. Display the poster in your classroom or on a projector. Alternatively, print it and use as a handout.
The accompanying activity uses a variety of elements and compounds on individual cards to investigate how bonding type can gradually change. The cards are not labelled with the name of the substance, so learners need to use their knowledge of structure and bonding to arrange them in order as they answer the questions.
Electronegativity
Electronegativity is a measure of the attraction between an atom involved in a bond and the electrons of that bond. The trends in electronegativity across the periods and down the groups of the periodic table can be explained in terms of covalent radius, nuclear charge and the screening effect of inner shell electrons.
Apart from simple covalent bonding between atoms of the same element, all bonds have some degree of covalent and ionic character. The larger the difference in electronegativities between bonded atoms, the more polar the bond will be and the greater the ionic character of the bond.
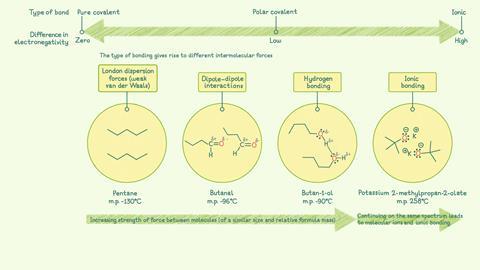
When the difference is very large, the movement of bonding electrons from the element of lower electronegativity to the element of higher electronegativity is complete, resulting in the formation of ions. Therefore, rather than isolated categories of bonding we have a bonding continuum.
Want more posters and activities on bonding?
Use this with your post-16 classes:
Try these with your 14–16 year-old learners:
Intermolecular forces
As the difference in electronegativity between the atoms in a bond goes from low to high, the strength of the forces between molecules increases. (Note: this explanation is limited to simple covalent bonding and does not include giant covalent molecules such as diamond, graphite or silica dioxide.)
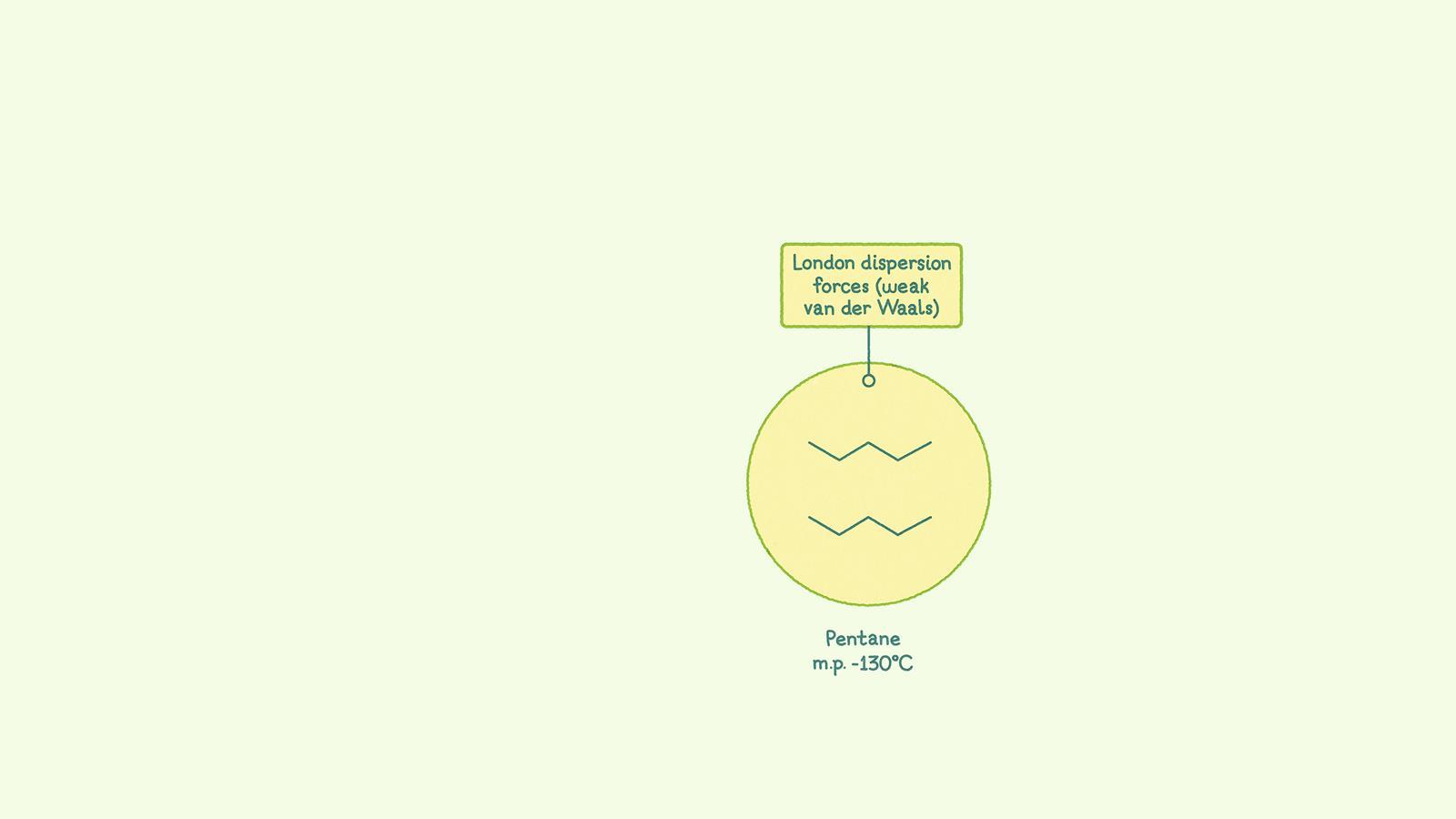
London dispersion forces, also known as Van der Waals forces, are the weakest intermolecular forces. They arise when the movement of electrons creates a temporary dipole in a molecule. This can then induce a dipole in a neighbouring molecule. The two dipoles are attracted to one another. These forces occur between molecules where there is very little or no difference in electronegativity between the atoms in a bond
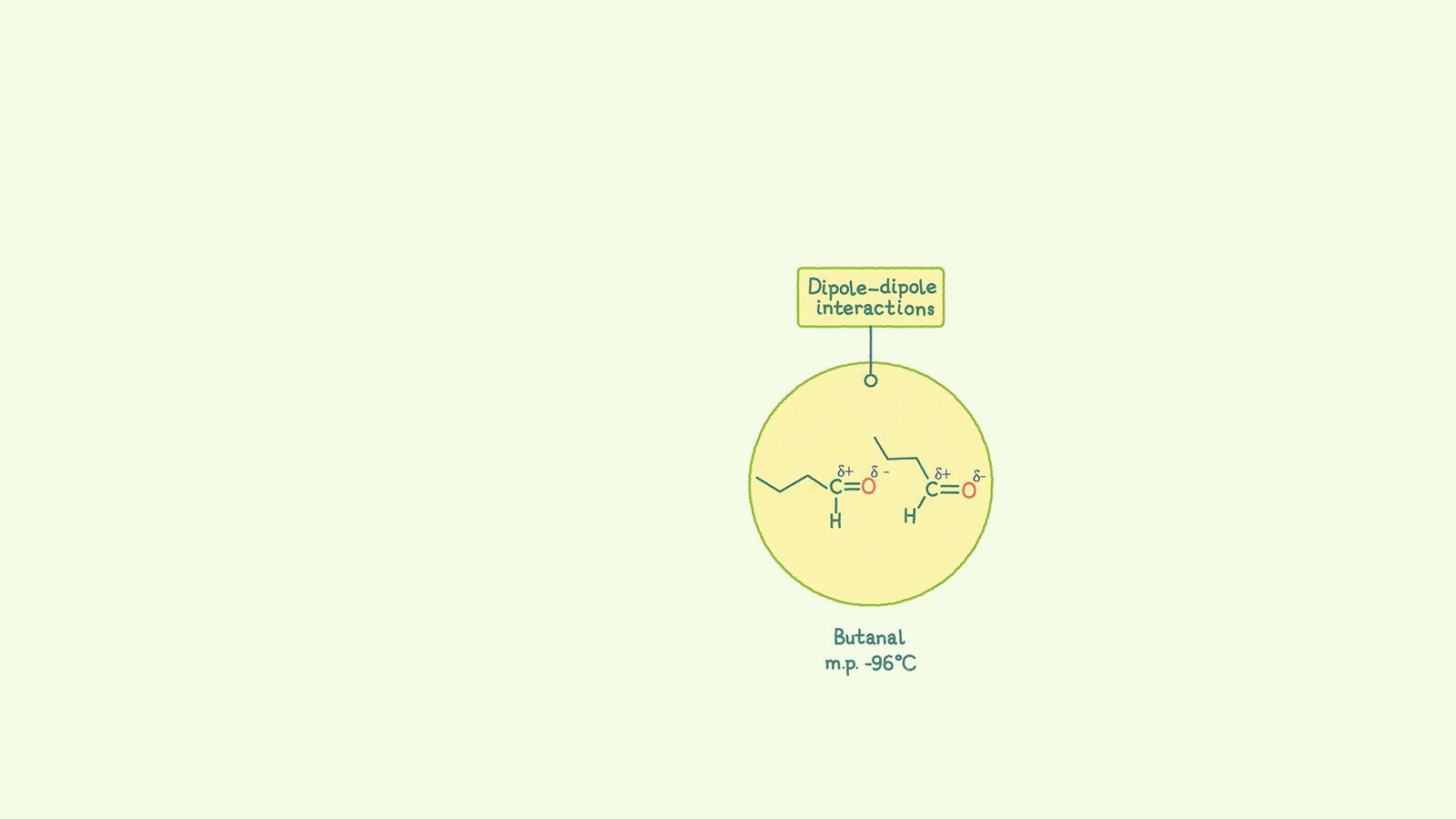
Permanent dipole–dipole forces occur between polar covalent bonds which are formed when the electronegativity of the bonding atoms is different. This results in an uneven distribution of charge, giving rise to a permanent dipole as atoms have partial charges (δ+)(δ-)
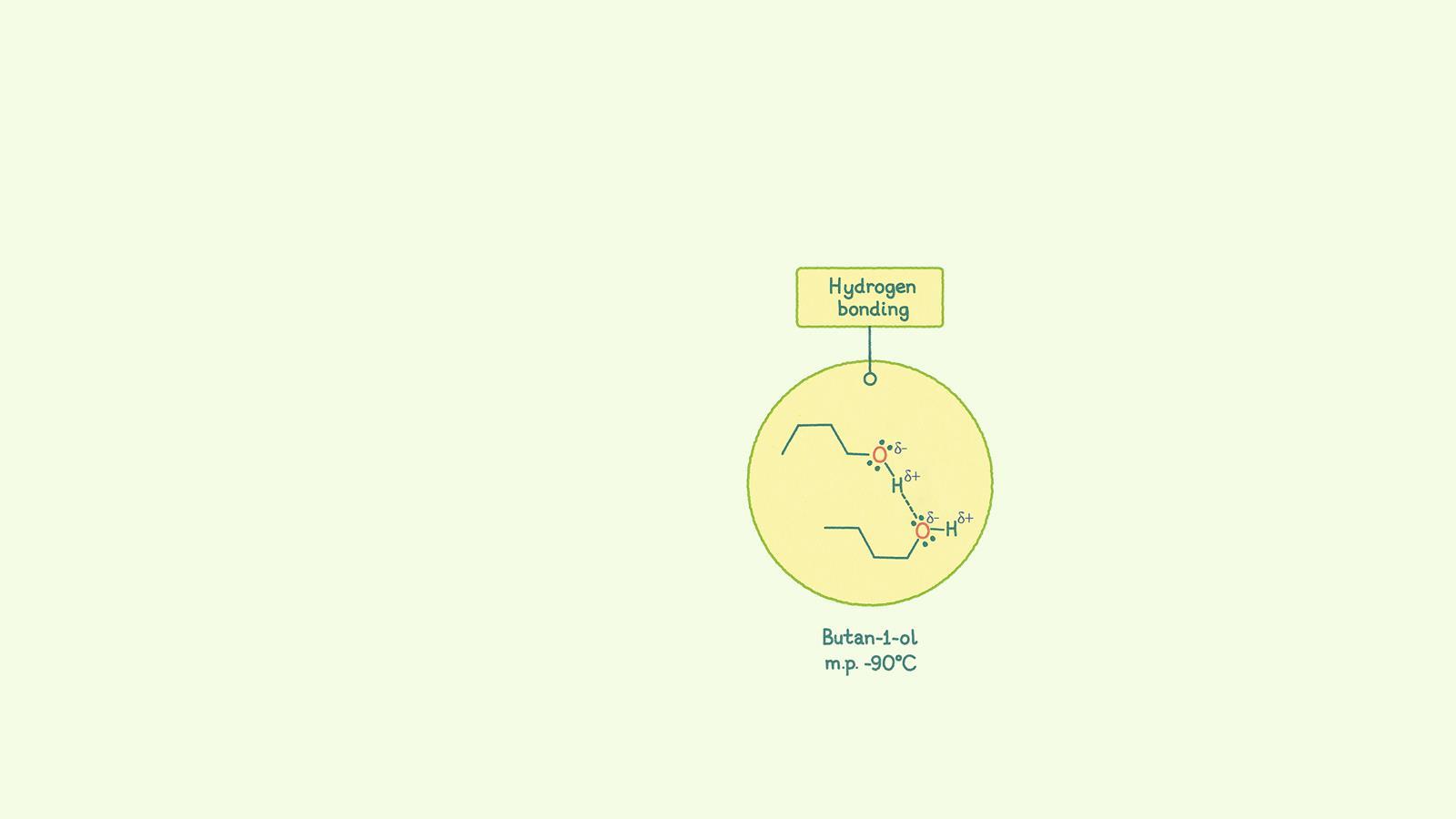
Hydrogen bonds form between molecules that have hydrogen atoms bonded to electronegative atoms, such as nitrogen, oxygen and fluorine which also contain a lone pair. These bonds are particularly strong as hydrogen atoms are very small and are attracted to the lone pair of electrons on the adjacent molecule
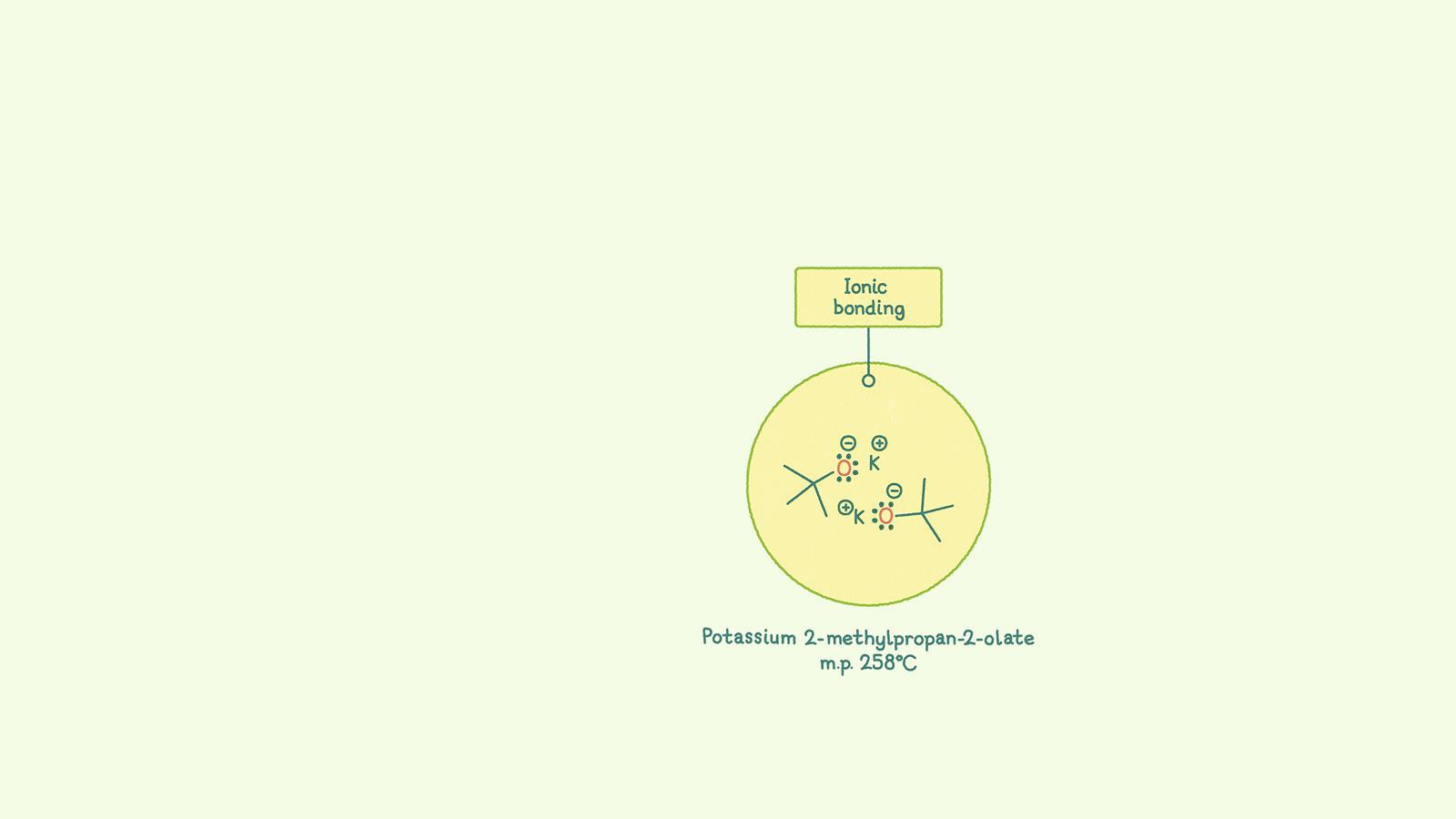
Ionic bonds are electrostatic attractions between positive and negative ions. The ions are arranged so the attractive forces between oppositely charged ions are stronger than the repulsive forces between same-charged ions
Did you know…?
Hydrogen bonding is responsible for holding together macromolecules such as DNA, proteins and cellulose. It also accounts for water’s unique, life-sustaining properties as well as explaining the different boiling points of isomeric amines.
All illustrations © Dan Bright
More resources
- Starter for ten quizzes that focus on bonding topics like the nature of chemical bonds, covalent bonding and properties and bonding.
- Try these tips, contexts and activities to tackle the orbital model of the atom and bonding with our post-16 CPD series.
- Use this Spot the bonding activity which asks learners to explore their knowledge of different bonding types, through diagrams of 18 bonds.
- Check students’ understanding of how properties of substances depend on their structure and bonding using this lesson plan with activities for 16–18 year olds.
This article was updated on 26 September to change the butan-1-ol structure.
Downloads
Spectrum of bonding resource - student worksheet
Handout | PDF, Size 0.46 mbSpectrum of bonding resource - teacher notes and answers
Handout | PDF, Size 0.18 mbSpectrum of bonding resource - student worksheet
Editable handout | Word, Size 0.79 mbSpectrum of bonding resource - teacher notes and answers
Editable handout | Word, Size 0.45 mbThe bonding spectrum fact sheet
PDF, Size 0.13 mbThe bonding spectrum fact sheet
Word, Size 0.45 mbThe bonding spectrum poster
PDF, Size 1.36 mb



















2 readers' comments