Ideas for enhancing chromatography practical work
Why study chromatography?
Using modern applications and contexts is a great way to hook your students and capture their interest. Look at figure 1 and think about what the images have in common.

Chromatography is the link: Chromatography Today is a great place to find interesting stories about chromatography and its applications to share with your students.
Chromatography is a key analytical technique students will meet several times during their secondary education (see the chromatography curriculum information sheet– a summary of the programme of study and subject content at key stages 3, 4 and 5). It also a required practical activity in most GCSE courses (table 1).
| Specification | Practical |
|---|---|
| AQA GCSE Chemistry (8462) | Required practical: 6 Investigate how paper chromatography can be used to separate and tell the difference between coloured substances. Students should calculate RF values. |
| Edexcel GCSE (9-1) Chemistry | 2.11 Core practical Investigate the composition of inks using simple distillation and paper chromatography. |
| OCR GCSE (9-1) Gateway Chemistry A | PAG C3 Using chromatography to identify mixtures of dyes in an unknown ink. |
| OCR GCSE (9-1) Twenty First Century Science Chemistry B | PAG 3 Using chromatography to identify mixtures of dyes in a sample of an unknown composition |
| WJEC Eduqas GCSE (9-1) Chemistry | Specified practical work: SP1B Separation of liquids by distillation and by paper chromatography |
How can we ensure there is a clear progression in the knowledge students develop? How can we maintain their interest and help them develop practical skills? How can we make chromatography in the classroom more engaging?
Underpinning chemistry and progression
The chemistry behind chromatography involves some quite basic concepts (figure 2) that are first introduced in primary schools and then revisited during key stage 3. These important ideas lay the foundations for understanding chromatography at key stage 4, when they can be further developed and applied to this technique.
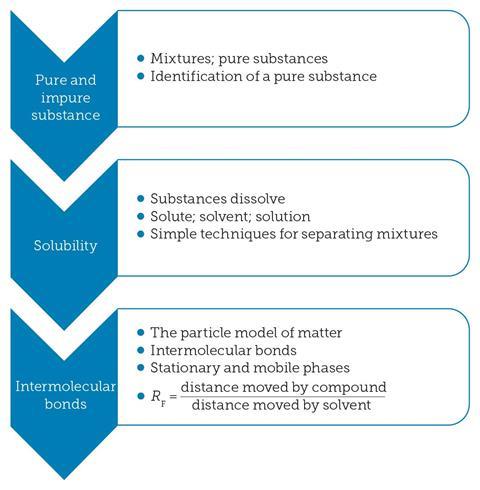
It is worth taking a moment to check your student’s understanding of the basic concepts (see table 2 for suitable resources) before launching into chromatography at key stage 4.
| Resource | Comment |
|---|---|
| What happens when something dissolves? |
Assessment for Learning is an effective way of actively involving students in their learning. This lesson plan comes with student worksheets and suggestions of how to organise activities |
| Mass and dissolving Elements, compounds and mixtures |
These activities provide strategies for dealing with some misconceptions students may have, in the form of ready-to-use classroom resources |
| Modern chemical techniques: chromatography | Use this book chapter to enhance your own knowledge of different types of chromatography |
| Thin layer chromatography Paper chromatography |
Further background information, including how to develop chromatograms so colourless spots become visible, and how to get better separation by using two-way chromatography |
| Table 2: Useful resources for checking student understanding or explaining how chromatography works | |
Developing practical skills and progression
There is a danger students will simply repeat the same chromatography experiment throughout their education. Popular activities with key stage 2 children include separating the mixtures found in felt-tip pens and sweets. At key stage 3 students separate the mixtures in inks and food colourings. Some exam boards specify the chromatography of inks and dyes as GCSE practical activities (table 1).
One of the challenges faced by teachers is how to develop students’ practical skills and maintain their interest if they are repeating experiments. There are several possible tactics: using different contexts; setting investigative or problem-solving activites; and taking a synoptic approach.
Another challenge with practical work is for students to understand what they are doing and why they are doing it. Getting this correct during lessons will help students answer practical-based exam questions. One tactic is to provide plenty of opportunities for students to do more than just follow a given procedure.

Synoptic approach
Example: the chromatography of pigments in leaves
Some possible questions to get your students started:
- What is the green pigment in leaves?
- Where have you come across it before?
- Where have you extracted chlorophyll before?
- How did you extract the chlorophyll?
- What techniques did you use?
- How could you carry out the chromatography experiment?
Planning activity
Explain how you would carry out each stage. Give details of the equipment needed and experimental procedures.

Practical problems and suggested solutions
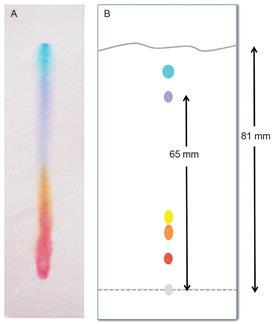
Chromatography is often seen as a quick and easy activity. However, the chromatograms students see in their textbooks and on exam papers often look nothing at all like the chromatograms from their own experiments. Typical examples are shown in figure 3.
So, faced with these results:
- How do we convince students they have carried out a similar process?
- Where is the centre of the spot?
- What value should the student use to calculate the RF value?
- How can you compare RF values with such big error bars?
Calculating RF
RF = Distance moved by compound / Distance moved by solvent
For the purple spot in figure 3b (the textbook diagram), the RF is 0.8. For the same spot in figure 3a (typical student results), what is the distance moved by the spot? Where would you draw the line? There will be a very large error in any value calculated.
Improving the results

Paper chromatography doesn’t give very good separation in classroom experiments. You would need a long piece of paper and lengthy elution time to get good separation. However, there are several things you can do to make improvements.
Changing the stationary phase by using thin layer chromatography (TLC) can lead to better separation. In figure 4, TLC gives clear bands with more easily measurable RF values: the solvent in both cases was a dilute solution of sodium chloride and the same black ink is used in both. Note the difference in the order in which the component dyes move up the stationary phase.
TLC is not covered in all GCSE specifications (see the curriculum information sheet, below) but it gives great results and the principles and underlying chemistry are the same as paper chromatography. The only real difference is the stationary phase, which may be silica on a polyester backing or aluminium oxide coated plates. The mobile phase remains the same but the way the two phases interact with the sample component is different.1
Figure 5 shows how changing the mobile phase, ie using different solvents, can also give better separation.
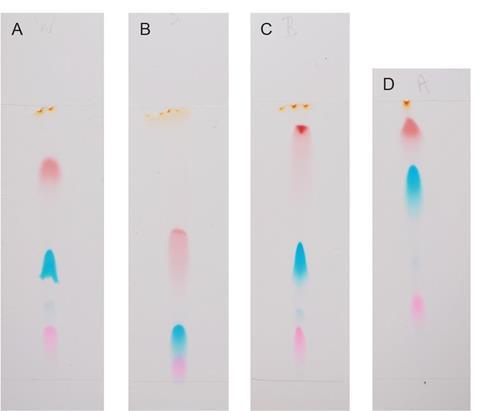
Changing the phases is the basis of a good investigation. The activity could be a problem solving exercise or competition, where students have to work out how to get good separation and then go on to produce the best chromatogram.
Hopefully your students will be able to accept that the chromatograms seen in figures 4b and 5 are a little closer to what they might meet in the exam (figure 3b).
Chromatography equipment

Although TLC plates are expensive, a classroom set of small plates can be cut from one standard plate. (Tip: If using right-handed scissors, try cutting with the backing side of the sheet uppermost. This seems to minimise flaking of the fragile coating. Alternatively, use a scalpel, ruler and cutting board.)
An expensive chromatography tank is not necessary to get good chromatograms. There are many methods that give good results. A one-holed boiling tube and crocodile clip (figure 6a) is useful for large classes and when volatile solvents (eg ethanol or propanone) are needed. Only small quantities of solvents are needed and fumes can be kept to a minimum.
A small vial and thin layer plate (figure 6b) can also give good separation over a very short distance. This small-scale approach is described by several sources, including these activities on plant pigments.
TLC plates with a fluorescing agent in the coating can be obtained (figure 6c). These are useful for locating colourless components – many colourless compounds absorb UV light and appear as darker spots.
Learn Chemistry resources
There are a number of chromatography resources on Learn Chemistry for students of all levels. Some provide further background information, such as how to link chromatography with particle theory and how chromatography is used in the workplace. Others are experiments ready to use with your students, for example the chromatography of leaves or sweets.
Download a table of learn chemistry resources, with ideas on how to include them in your teaching, below.
Kay Stephenson and Dorothy Warren are independent science education consultants. Figures 3, 4, 5, and 6 courtesy of Kay Stephenson
Downloads
Resources on Learn Chemistry
PDF, Size 51.83 kbResources on Learn Chemistry
Word, Size 53.34 kbCurriculum information
PDF, Size 88.72 kbCurriculum information
Word, Size 60.94 kb
References
- B Faust, Modern chemical techniques, p119. Royal Society of Chemistry, 1997 (rsc.li/2kmh457)









No comments yet