Explore dissolving and conservation of mass, tackle common misconceptions
Younger learners may be satisfied with the idea that solutes ‘disappear’ when they dissolve. Even when the process of dissolving is appreciated, learners may not expect the mass of the solute to register in any measurements.
-

Download this
Encourage learners to observe, think about and explain the process of dissolving with this worksheet, complete with teacher guidance.
View and download more Chemical misconceptions resources
How to use this resource
Use this exercise to ask learners to predict the masses of solutions from given masses of solute and solvent, to explain what happens to the solute and the emergent properties of the solution.
Read the chapter Chemical axioms, to find out more about learners’ beliefs in alternative ideas.
- When to use? This exercise is primarily aimed at the 11–14 age range, although you can use it to check the understanding of your 14–16 learners. Use this task as an introductory diagnostic activity before formally teaching about the topic at either level.
- Group size? Suitable for independent work in class to diagnose learners’ misconceptions.
- Topics assessed? Conservation of mass during dissolving.
- How long? 10–15 minutes
Sugar and water
Some water was placed in a beaker, and its mass was measured using a balance. The mass of beaker and water was 200 g. Then 10 g of sugar was weighed out. The sugar was added to the water, and sank to the bottom. 10 minutes later the sugar could not be seen.
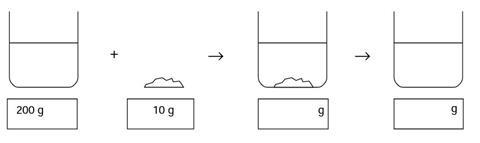
- Fill in the boxes to show what you think the mass of the beaker and its contents would be when the sugar was first added, and then after it could no longer be seen.
- Where did the sugar go? Explain your answer.
Salt and water
Some water was placed in a beaker, and its mass was measured using a balance. The mass of beaker and water was 150 g. Then 10 g of salt was weighed out. The salt was added to the water, and sank to the bottom. 10 minutes later the salt could not be seen.
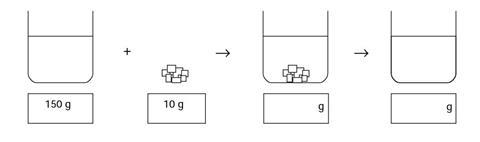
- Fill in the boxes to show what you think the mass of the beaker and its contents would be when the salt was first added, and then after the salt could no longer be seen.
- Where did the salt go?
Copper sulfate and water
Some water was placed in a beaker, and its mass was measured using a balance. The mass of beaker and water was 250 g. Then 5 g of blue crystals of copper sulfate was weighed out. The copper sulfate was added to the water, and sank to the bottom. 20 minutes later the copper sulfate could not be seen, but the liquid had turned blue.
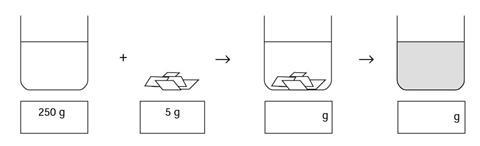
- Fill in the boxes to show what you think the mass of the beaker and its contents would be when the copper sulfate was first added, and when it could no longer be seen.
- Why did the water turn blue?
- Where did the copper sulfate go?
Particles in sugar and water
The diagrams below represent the particles present at the different stages when sugar is dissolved in water. Not all the particles are shown.
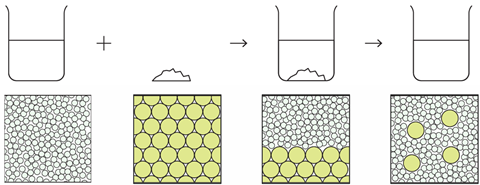
Why does the liquid taste sweet when sugar is added to water?
Lesson plan also available
Build a lesson around this resource with our What happens when something dissolves? lesson plan. Ask learners to observe and think about the process of dissolving and use their knowledge of the particle model to explain their observations.
Download as part of a complete lesson with presentation slides.
Answers
Sugar and water
- 210 g, 210 g
- The sugar dissolved - it is still present, but as part of the solution. The molecules of sugar, which are much too small to be seen, are mixed with the molecules of water.
Guidance note: When this exercise was piloted in schools it was found that some learners did not expect mass to be conserved on dissolving (even though most recognised that the solute was still present in some form), and that learners who conserved mass in their responses often had only vague ideas about how the properties of solutions arose.
Salt and water
- 160 g, 160 g
- The salt dissolved, and is now part of the solution. The salt particles are mixed with the water particles in the solution.
Guidance note: If you are using this resource with older learners who have studied ionic bonding, emphasise that the sodium ions and chloride ions are separate in the mixture.
Copper sulfate and water
- 255 g, 255 g
- The blue colour is a property of the particles in the copper sulfate. The water turned blue as the copper sulfate dissolved to give a solution. The copper sulfate particles are mixed with the water particles. As the copper sulfate particles are spread throughout the solution the whole solution looks blue.
- The copper sulfate dissolved - it is still present, but as part of the solution. (The particles of copper sulfate, which are much too small to be seen, are mixed with the molecules of water.)
Guidance note: Learners commonly believe that the properties of a substance are due to its particles having that same property (read more about learners’ beliefs in alternative ideas). This idea is usually not correct, so although in this case the colour may be seen to be a property of both the particles and the bulk material, emphasise that this is unusual, and that most bulk properties are not shared by the particles.
If learners have studied ionic bonding, emphasise that the copper ions and sulfate ions are separate in the mixture. The colour is due to the hydrated copper(II) ions.
Particles in sugar and water
The liquid tastes sweet because the molecules of sugar are dissolved in the solution. Sugar has a sweet taste, so the solution tastes sweet because it contains the sugar molecules.
Downloads
Mass and dissolving student sheet
Handout | PDF, Size 0.32 mbMass and dissolving teacher notes
Handout | PDF, Size 0.2 mbMass and dissolving student sheet
Editable handout | Word, Size 0.64 mbMass and dissolving teacher notes
Editable handout | Word, Size 0.59 mb
Additional information
These resources have been taken from the book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 2, by Keith Taber.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
 Currently
reading
Currently
reading
Mass and dissolving | 11–14 years
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36






































































































No comments yet