A masterclass in teaching the topic of bonding, basing chemical explanation on physical forces
- Students can hold the common misconception of the dichotomy of ionic and covalent bonds
- Considering a quantum theory of matter helps to underpin a more advanced understanding of bonding
Chemical bonding is a topic found challenging by students, who commonly develop alternative conceptions which act as impediments to learning the scientific models.1,2 This article offers an introductory treatment of the topic. It is intended to support teaching that avoids encouraging the common misconceptions, and which supports progression through the secondary/college years. In particular, chemical bonding is here understood as part of a more general way of modelling the structure of matter at the scale of molecules.3
Space in this article only allows a cursory exploration of basic bonding ideas, but this should be sufficient for readers to get a feel for the strengths and weaknesses of this approach. Much of this presentation is supported by research into how students' ideas develop, and the misconceptions they often develop with current common approaches to teaching the topic.
A theoretical notion
Chemical bonding is one of the key concepts in the study of chemistry, and is important in understanding material structures, properties and reactions. Yet the notion of the chemical bond is purely theoretical, in the sense that it does not refer directly to any readily observable phenomenon. Rather the idea of the chemical bond is part of a conceptual framework developed by chemists to explain phenomena that can be observed in the laboratory in terms of an elaborate set of models of matter at a submicroscopic scale.
The key point of chemists' submicroscopic models that novices commonly fail to appreciate is that the macroscopic properties and behaviours of substances are explained in terms of the distinct properties of the submicroscopic entities.4 Unfortunately it is common for learners to misconstrue how these explanatory models work, and to think that the properties of substances are explained by assuming they are composed of tiny particles having those properties: eg that copper conducts because it is made up of copper atoms that are themselves conducting.
Matter binds together
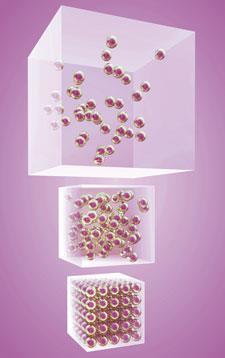
A basic premise of the explanatory framework constructed by chemists is that apparently solid, continuous, matter is actually discontinuous and composed of myriad tiny components. That is, chemists use a quantum theory of matter. I will refer to the various components as 'quanticles'. Although these quanticles can be imagined as tiny particles, a bit like specks of dust or grains of salt, that is misleading as they have some properties very unlike familiar particles (like being impossible to localize in space, sometimes interpenetrating each other, and - under certain conditions - giving rise to interference patterns).
A key feature of these quanticles is that because some of them are charged, matter tends to bind together, and this is the basis of the explanation for why matter on the more familiar scale is held together. Unfortunately when 'particle theory' is first introduced in secondary schools, usually to explain the three states of matter, the emphasis on the arrangement of the 'particles' in solids, liquids and gases often leads to this key property being neglected, leaving learners with little rationale for why the condensed phases exist.5
The central principle that matter binds together, but the bonding can be disrupted at high enough temperatures, applies at a range of levels (ie lattices, molecules, atoms, nuclei, hadrons) and can be introduced in the context of understanding why there are different states of matter, and why phase changes occur in association with heat flow into or away from the substance.
The most fundamental quanticles, for chemists at least, are the proton, neutron and electron, although other important quanticles are formed when these bind together. The protons and neutrons, collectively known as nucleons, can bind together through the strong nuclear force to produce aggregates termed nuclei. For most chemical purposes these aggregates can be considered stable.
Positively charged nuclei attract negatively charged electrons, so under normal conditions nuclei are surrounded by a swarm or 'cloud' of electrons, such that they tend to produce overall neutral, or nearly neutral, conglomerates. The interactions between the individual charged quanticles can be considered to be coulombic forces: that is if we model any two charged quanticles as point charges, the force between them is proportional to the product of the charges and inversely proportional to the square of their separation:
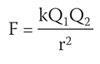
where F refers to force, k is a constant, r represents separation, and Q indicates charge.
This equation can be applied in principle to the interactions between two nuclei, a nucleus and an electron, two ions, etc. (It is not always appropriate to consider quanticles as if point charges, although this assumption is useful in many contexts. Whilst the formula will not be used quantitatively in introductory chemistry, it is useful that students are asked to think about the electrical nature of the forces.) Most systems of interest contain more than two charges, and the net force on a particular quanticle will be the vector sum of all the F values calculated from all its interactions.
This electrostatic model can be used as the basis of explaining a great deal of chemistry. Stable structures are in an equilibrium where the various forces balance out. In all the examples of bonding below, quanticles are attracted together until they reach a point where they are close enough for repulsions to balance attractions.
However, at the submicroscopic scale, it is not only matter that is quantized - so for example, are energy and momentum. This leads to constraints on the configurations that quanticles can take up. In terms of the simplest models of matter at this level, this means that the arrangement of electrons associated with nuclei follow certain patterns, and these are often described in terms of electrons forming 'shells' where only two electrons can occur in the first shell (nearest the nucleus), but the next can accommodate eight, and the next eighteen.
The notion of a 'shell' is a metaphoric one: the electrons moving around the nucleus can be considered to provide a sphere of charge that surrounds the nucleus. Just as electrons and nuclei can be considered to act as point charges, so for many purposes can complexes of nuclei surrounded by shells of electrons.
Building up more complex quanticles

The materials on earth today are considered to have been formed in the furnace of a large star, which then exploded. Inside the star the temperature would have been great enough for matter to exist as plasma: a kind of gas comprised of bare nuclei and free electrons. Later as the ejected material cooled, there would not be enough energy to overcome the electrical binding and electrons would have been attracted to the nuclei and started to bind around them in shells. A nucleus of carbon, with a charge of +6 would have repelled other nuclei it came near, but attracted electrons. As the temperature fell it would bind two electrons into a shell around it. The new structure, a carbon 4+ ion, would have then remained stable until the material cooled further (because the its overall charge was reduced and further electrons would only be attracted into the next shell, further from the nucleus).
As the temperature continued to fall, matter tended to settle into neutral combinations of quanticles. Many students would assume that the result of this process would be the production of neutral atoms of chemical elements (which they commonly consider to be the entities involved in chemical reactions).6 However, if we consider the materials on earth today, only a few substances exist as atoms - the noble gases - and they do not take part in much chemistry.
We do not normally teach students about this account, which is unfortunate, as they tend to think of the states of substances they experience as being natural for that substance: eg iron is solid, nitrogen is gas. Appreciating that the strength of bonding in substances varies, but that in any substance the bonding will eventually be disrupted by increasing the energy of the component quanticles, is an important principle that can be understood by lower secondary pupils.7
Metallic bonding
Some metals are found 'native', but these are not atomic, but rather consist of extensive lattices comprising atomic cores with enough delocalised electrons moving around them to give an overall neutral structure. The cores are the nuclei surrounded by one or more shells of electrons, held in the lattice by the electrical forces between the positive cores and the delocalised electrons. A consequence of this type of bonding, metallic, is the conductivity (electrical, but also thermal) associated with metals. Other properties of metals can also be related to the bonding and structure at the level of quanticles.
Covalent bonding
Other elements found native, like sulfur, oxygen, and nitrogen, are not metals. Their basic components at the quanticle level are comprised of a small number of cores surrounded by a sheath of sufficient electrons to give overall neutrality. These arrangements are called molecules.
In our simple model we find that in molecules there are electrons between any two cores - so that the attraction between electrons and cores balances the repulsion between the cores, and holds the structure together. This is called covalent bonding. Sometimes it is said that electron pairs are 'shared' between cores, but this figurative language can be taken a little too seriously by learners who will see the sharing - rather than the balance of electrical forces - as the explanation for the bond.
In the case of oxygen and nitrogen, hydrogen, fluorine and chlorine, the molecules each comprise two atomic cores with the surrounding sheath of electrons. However phosphorus often consists of molecules with four cores (arranged as the corners of a tetrahedron), and sulfur molecules consists of eight cores arranged in a ring - with each core having a covalent bond with each of its neighbours in the ring.
In some structures, such as diamond, there are extensive networks of cores linked through covalent bonding so that there is a giant structure, although the individual bonds comprise of electron pairs localised between adjacent cores.
Intermolecular bonding
Phosphorus and sulfur are solids at room temperature, because the individual molecules are themselves bound together. On our simple model this can be explained in terms of the movements of electrons in the molecules leading to transient areas of positive and negative charge, which influence electrons distribution in adjacent molecules. The synchronisation of the transient shifts in charge leads to an attraction between molecules that attracts them together. This form of intermolecular bonding is an induced-dipole-induced-dipole interaction. Even the noble gases will condense due to these weak attractions when the temperature is low enough.
Polar bonding
Most material on earth is not in the form of elemental substances, and indeed there are a vast number of compounds, when the basic quanticles involved are cores of several different elements bound together. In covalent bonding, the electrons found between cores of the same atom are equally attracted towards both. However, when electrons are binding cores with different charges, the electron charge density pattern will reflect the different core charges.
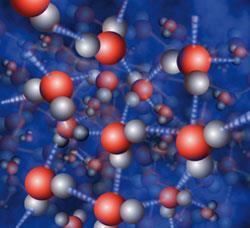
So, for example, in water molecules, H2O, the electron pairs between the hydrogen and oxygen cores are attracted more strongly to the oxygen cores (with their +6 charge) and so the 'average' position of the bonding electrons can be considered to be shifted towards the oxygen core. This type of bonding is called polar bonding. This gives molecules that have an asymmetrical distribution of charge and are themselves often polar. These polar molecules can align as the positive areas on one attract the negative areas of another (and vice versa).
Such permanent dipole interactions form another class of intermolecular bonding. This is particularly strong in water, and other compounds with hydrogen bonded to electronegative elements, when it is called hydrogen bonding. (Understanding the special nature of hydrogen bonding requires more sophisticated models.)
Ionic bonding
Some compounds have a different kind of structure that is not molecular, but rather a crystalline lattice. This ionic structure is rather different to the metallic lattice, and leads to different properties. Substances with ionic bonding do not conduct electricity in the solid state, and can often be dissolved in solvents consisting of polar molecules.
Ionic bonding tends to occur between electropositive metals and electronegative non-metals. Simple ions are single atomic cores surrounded by shells of electrons (whereas molecular ions contain several cores). Cations are cores of metallic elements, as found in metallic lattices. However, some nonmetallic elements will form anions where 'excess' electrons give the ions net negative charge. Individual ions are not usually stable as discrete entities as their charges lead to them interacting with other species. In ionic compounds each cation is surrounded by several anions, and vice versa.
The interspersing of positive and negative ions allows the ions to come close together until the repulsions balance the attractions. The bonding is the attraction between the ions and their 'counter-ions' in the lattice. So for example, in sodium chloride, each ion is surrounded by six oppositely charged ions and is equally 'bonded' to each. Students often find this difficult to grasp, as they tend to think that the charge on an ion should limit how many bonds it can form, so often assume each ion in NaCl only forms one 'proper' bond.
Solvation
As ions are charged they can be attracted to the appropriate ends of polar molecules. As bonds are just attractions between charges these interactions are a kind of bonding. Sometimes the solvation forces are sufficient to break down an ionic lattice. Similarly, molecular materials can also dissolve, although not in polar solvents (as the polar molecules are more strongly attracted to each other). However, sometimes a polar molecule can induce a charge distortion in a normally non-polar molecule - another flavour of intermolecular bonding.
Bonding continua
The approach to discussing the main categories here shows that covalent, polar and ionic bonding are modifications of the same basic pattern, as is metallic bonding. In each case positive cores are bound together by their attraction with negative electrons (Fig 1) - although in the ionic case those bonding electrons are effectively around the anions.
There are no 'pure' ionic substances, but different degrees of polarity distort the bonding away from the symmetrical arrangement of bonding electrons in the covalent model to different degrees. (In contrast, students commonly see bonding primarily in terms of a dichotomy, with ionic and covalent bonds fundamentally different - and polar bonding as a subtype of the covalent case).
Similarly, in considering, say, methane, benzene, graphite, and copper, we see how covalent bonding and metallic bonding are related (in terms of the degree of electron localisation), although this needs to be developed using molecular orbital ideas.
Chemical reactions
Chemical reactions involve the interactions of the types of systems discussed above, leading to rearrangements of the atomic cores and associated electrons to form new arrangements that are more stable overall. We can think of this as finding ways of jiggling the electrons into better positions to hold the cores together. This is a simplistic explanation: but it has a sound physical basis, unlike the very common misconception acquired by many students that reactions occur to allow atoms to fill up their shells.
As the model discussed here makes clear, very few materials exist as discrete atoms - and those few exceptions do not readily undergo chemical change.
Limitations and developments
Space here has only allowed brief discussion of a very basic model of chemical bonding. It is a sound model, up to a point, and can explain a lot. However, it has limitations, and when further explanatory power is needed it must be supplemented by more advanced models.
The framework here offers a number of advantages for teachers. It provides an intellectually honest set of simplifications that (a) undermines common misconceptions and (b) can underpin more advanced understanding of bonding and related topics such as reactions, ionisation, and chemical stability. Most importantly, it bases chemical explanation on physical forces, whereas with traditional teaching approaches most students come to see the molecular world in terms of the actions of atoms trying to satisfy needs.
Further Reading
1. K S Taber and R K Coll, Chemical Bonding, in Chemical Education: Research-based Practice , pp 213-234 Dordrecht: Kluwer Academic Publishers BV, 2002
2. A Hofstein et al, Stud. Sci. Educ., 2010, 46, 179
3. K S Taber, Chem. Educ. Res. Pract. Eur., 2001, 2, 123
4. J K Gilbert, D F Treagust, Chemical Education: Linking the representational levels of chemistry. Dordrecht: Springer, 2009
5. P M Johnson, Res. Sci. Educ., 2005, 35, 41
6. K S Taber, Int. J. Sci. Educ., 1998, 20, 597
7. P Johnson, Introducing Particle Theory. In Teaching Secondary Chemistry, 2nd edition. Association for Science Education/John Murray: Forthcoming









No comments yet